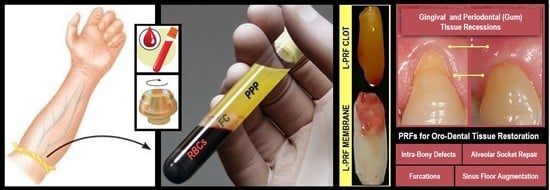
The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. PRFs in the Periodontal Intra-Bony Defects (IBDs) Treatment
3.2. PRFs in the Periodontal Furcation Defects (PFDs) Treatment
3.3. Miller Class I and II Gingival Recession Treatment by PRFs
3.4. PRFs in Sinus Floor Augmentation
3.5. PRFs in Alveolar Ridge Preservation
3.6. Clinical Expertise with L-PRF in Periodontally Accelerated Osteogenic Orthodontics
4. Conclusions and Closing Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Habibovic, P.; de Groot, K. Osteoinductive biomaterials—Properties and relevance in bone repair. J. Tissue Eng. Regen. Med. 2007, 1, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S. NanoDentistry: Perspectives on the Role of NanoBiotechnology in Biomaterials, Pharmaceutics and BioDental Tissue Engineering. EC Dent. Sci. 2015, 3, 506–507. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr. Pharm. Biotechnol. 2012, 13, 1231–1256. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2006, 101, e51–e55. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan Ehrenfest, D.M.; Everts, P.A.; Wiczkowski, A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: New perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Thorat, M.; Pradeep, A.R.; Pallavi, B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial: Platelet-rich fibrin and periodontal regeneration. J. Clin. Periodontol. 2011, 38, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Rosamma Joseph, V.; Raghunath, A.; Sharma, N. Clinical effectiveness of autologous platelet rich fibrin in the management of infrabony periodontal defects. Singap. Dent. J. 2012, 33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A.R. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: A randomized controlled clinical trial. J. Periodontol. 2011, 82, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects: Xenograft and platelet-rich fibrin in intrabony defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Bansal, C.; Bharti, V. Evaluation of efficacy of autologous platelet-rich fibrin with demineralized-freeze dried bone allograft in the treatment of periodontal intrabony defects. J. Indian Soc. Periodontol. 2013, 17, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, Y.; Uraz, A.; Taner, I.L.; Çulhaoglu, R. Effects of platelet-rich fibrin on healing of intra-bony defects treated with anorganic bovine bone mineral. Braz. Oral Res. 2017, 31, e15. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sankari, M.; Satpathy, A.; Jayakumar, D.; Mozzati, M.; Mortellaro, C.; Gallesio, G.; Taschieri, S.; Del Fabbro, M. Adjunctive Effect of Autologus Platelet-Rich Fibrin to Barrier Membrane in the Treatment of Periodontal Intrabony Defects. J. Craniofac. Surg. 2016, 27, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A.R. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: A randomized clinical trial. J. Periodontol. 2011, 82, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Padma, R.; Shilpa, A.; Kumar, P.A.; Nagasri, M.; Kumar, C.; Sreedhar, A. A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller’s class-I and II recession defects. J. Indian Soc. Periodontol. 2013, 17, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Aroca, S.; Keglevich, T.; Barbieri, B.; Gera, I.; Etienne, D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J. Periodontol. 2009, 80, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, S.; Aleksic, Z.; Milinkovic, I.; Dimitrijevic, B. The coronally advanced flap in combination with platelet-rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: A comparative study. Eur. J. Esthet. Dent. 2010, 5, 260–273. [Google Scholar] [PubMed]
- Eren, G.; Atilla, G. Platelet-rich fibrin in the treatment of localized gingival recessions: A split-mouth randomized clinical trial. Clin. Oral Investig. 2014, 18, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, S.; Aleksic, Z.; Klokkevold, P.; Lekovic, V.; Dimitrijevic, B.; Kenney, E.B.; Camargo, P. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int. J. Periodontics Restor. Dent. 2012, 32, e41–e50. [Google Scholar]
- Zhang, Y.; Tangl, S.; Huber, C.D.; Lin, Y.; Qiu, L.; Rausch-Fan, X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J. Craniomaxillofac. Surg. 2012, 40, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gassling, V.; Purcz, N.; Braesen, J.H.; Will, M.; Gierloff, M.; Behrens, E.; Açil, Y.; Wiltfang, J. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: A preliminary study. J. Craniomaxillofac. Surg. 2013, 41, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Niu, Z.; Xue, Y.; Yuan, F.; Fu, Y.; Bai, N. Improvement in the repair of defects in maxillofacial soft tissue in irradiated minipigs by a mixture of adipose-derived stem cells and platelet-rich fibrin. Br. J. Oral Maxillofac. Surg. 2014, 52, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P. Focus on intrabony defects—Conservative therapy. Periodontol. 2000 2000, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Ross, I.F.; Goteiner, D. Effect of periodontal therapy on patients maintained for 15 years or longer. A retrospective study. J. Periodontol. 1986, 57, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, P.; Prato, P.G. Coronally advanced flap and combination therapy for root coverage. Clinical strategies based on scientific evidence and clinical experience. Periodontol. 2000 2012, 59, 158–184. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar] [PubMed]
- Cordaro, L. Bilateral simultaneous augmentation of the maxillary sinus floor with particulated mandible. Report of a technique and preliminary results. Clin. Oral Implants Res. 2003, 14, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, J.P.; ten Bruggenkate, C.M.; Krekeler, G.; Tuinzing, D.B. Sinusfloor elevation and grafting with autogenous iliac crest bone. Clin. Oral Implant. Res. 1998, 9, 429–435. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Araújo, M.G.; Simion, M.; Osteology Consensus Group 2011. Evidence-based knowledge on the biology and treatment of extraction sockets. Clin. Oral Implant. Res. 2012, 23, 80–82. [Google Scholar] [CrossRef]
- Pripatnanont, P.; Nuntanaranont, T.; Vongvatcharanon, S.; Phurisat, K. The primacy of platelet-rich fibrin on bone regeneration of various grafts in rabbit’s calvarial defects. J. Craniomaxillofac. Surg. 2013, 41, e191–e200. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, F.; Jiménez, C.; Espinoza, D.; Vervelle, A.; Beugnet, J.; Haidar, Z. Use of leukocyte and platelet-rich fibrin (L-PRF) in periodontally accelerated osteogenic orthodontics (PAOO): Clinical effects on edema and pain. J. Clin. Exp. Dent. 2016, 8, e119–e124. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kohli, M.; Gupta, N. Platelet Rich Fibrin: A Novel Approach for Osseous Regeneration. J. Maxillofac. Oral. Surg. 2012, 11, 430–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, F.; Haidar, Z. L-PRF for Use in Oro-Maxillo-Facial Surgeries: What Do We Know? J. Oral Res. 2018, 7, 88–90. [Google Scholar] [CrossRef] [Green Version]




| Application | No. Patients/Defects | Groups | Follow-up (Months) | Main Finding(s) | Reference |
|---|---|---|---|---|---|
| IBD | 32/32 | (1) PRF + open flap surgery (2) Open flap surgery | 9 | All sites healed uneventfully. Probing depth (PD) reduction, average clinical attachment (CAL) gain, defect fill, percentage defect fill and post-treatment gingival margin stability were significantly greater in the PRF-treated group. (P < 0.05). | [9] |
| IBD | 15/30 | (1) PRF + open flap surgery (2) Open flap surgery | 12 | All sites healed uneventfully. PD reduction, CAL gain, radiographic IBD depth reduction, and post-treatment gingival margin stability were significantly higher in the PRF group. Statistically significant higher patient acceptance and healing index in PRF vs. control. | [10] |
| IBD | 35/56 | (1) PRF + open flap surgery (2) Open flap surgery | 9 | All sites healed uneventfully. PD reduction, CAL gain, radiographic IBD defect fill were significantly higher in the PRF group. Gingival Margin Stability (GMS) was higher in the PRF group. | [11] |
| IBD | 17/34 | (1) PRF + Bio-Oss® (2) PRF | 6 | All sites healed uneventfully. Both groups showed significant PD reduction, CAL gain, and IBD fill. Intergroup differences were also significant and favored the PRF/Bio-Oss group. | [12] |
| IBD | 10/20 | (1) PRF + DFDBA (2) DFDBA (demineralized-freeze dried bone allograft) | 6 | Both groups experienced significant PD reduction, CAL gain, IBD fill, and IBD resolution. Intergroup differences were statistically significant only for PD reduction and CAL gain, favoring the PRF/DFDBA group. | [13] |
| IBD | 21/21 | (1) PRF + inorganic bovine bone mineral (2) Anorganic bovine bone mineral | 6 | All of the sites healed uneventfully with no clinically detectable or subjectively reported side effects. Both treatment groups showed significant improvements compared to baseline in terms of vertical bone gain, defect fill, and defect angle at six months after treatment (P < 0.05). Addition of PRF to inorganic bovine bone mineral (ABBM: Anorganic bovine bone mineral) may lead to the enhancement of clinical attachment level gain. | [14] |
| IBD | 16/32 | (1) Resorbable collagen membrane + PRF (2) Guidance tissue regeneration | 9 | Test group showed a statistically significant improvement for probing depth (P = 0.002), clinical attachment level (P = 0.001), and radiographic defect depth (P < 0.001) after nine months as compared with the control sites. The adjunctive use of PRF in combination with barrier membrane is more effective in the treatment of intrabony defects in chronic periodontitis as compared with barrier membrane alone. | [15] |
| PFD (Periodontal Furcation Defect) | 18/38 | (1) PRF + open flap surgery (2) Open flap surgery | 9 | All sites healed uneventfully. No significant visual differences between groups were noticed. Complete clinical closure was achieved in 66.7% of the defects in the PRF group. Within residual furcation defects, 5/6 were reduced from grade II to grade I, and one defect remained grade II. Significantly greater PD reduction, CAL gain, and defect fill was noticed in the PRF-treated group vs. control. | [16] |
| Gingival Recession | 15/30 | (1) PRF + coronally-advanced flaps (CAF) (2) CAF | 6 | Both groups experienced statistically significant recession depth (RD) reduction, CAL gain, and keratinized tissue width (KTW) increase at all time intervals (P < 0.05). Intergroup differences were statistically significant and favored the PRF group. Mean percentage of root coverage for the test and control groups were 100% and 68.44%, respectively. Differences between groups were statistically significant and favored the PRF group. | [17] |
| Gingival Recession | 20/67 | (1) PRF + CAF (2) CAF | 6 | With the exception of CAL gain and gingival tissue thickness (GTH) increase, the addition of PRF to CAF failed to produce significant additional clinical benefits (vs. CAF-alone). Percentage root coverage, full root coverage, GMS, and recession width (RW) reduction were significantly higher in the CAF controls than the PRF-treated sites after six months. | [18] |
| Gingival Recession | 20/40 | (1) PRF + CAF (2) EMD + CAF | 12 | Both groups experienced statistically significant RD reduction, PD reduction, and KTW increase. Intergroup differences were significant only for KTW increase and favored the enamel matrix derivate (EMD) group. Mean root coverage was 70.5 ± 11.76% in the EMD group, and 72.1 ± 9.55% in the PRF group. Complete root coverage was achieved in 60% of the EMD sites and 65% of the PRF sited. No intergroup comparison was carried out. The healing index of the PRF group after the first week was significantly superior to that of EMD. Non-significant differences between groups were found after two weeks post-surgery. Three patients of the EMD group and one of the PRF group experienced severe pain. All of the patients in the EMD group reported greater discomfort. Analysis of the first five days post-surgery revealed statistically significant differences between both groups favoring PRF (less pain). | [19] |
| Gingival Recession | 22/44 | (1) PRF + CAF (2) SCTG(subepithelial connective tissue graft) + CAF | 6 | Both groups experienced a statistically significant decrease in RD (Gingival recession depth), RW (Gingival recession width), and RA (Gingival Recession Area), plus an increase in CAL (Clinical Attachment Level) gain, KTW (Keratinized Tissue Width), and GT (Gingival Thickness). Intergroup differences were non-significant. Higher yet non-significant gingival margin stability was reported for the PRF group. Percentage of root coverage and complete root coverage were 92.7% and 72.7% in the test group and 94.2% and 77.3% in the control group. No statistical significant differences between both groups were found (P > 0.05). All of the sites healed uneventfully; however, the control group reported complications (i.e., pain) related to the palate donor site. | [20] |
| Gingival Recession | 15/30 | (1) PRF + CAF (2) Connective tissue grafts (CTG) + CAF | 6 | Both groups experienced a significant CAL gain, RD reduction, and GMS. Intergroup differences were non-significant. Both groups experienced a statistically significant increase in KTW. Intergroup differences were statistically significant and favored the CTG group. Mean root coverage was 88.68 ± 10.65% for the PRF group and 91.96 ± 15.46% for the control group. Complete root coverage was achieved in 75.85% of cases in the PRF group and 79.56% of cases in the control group. Intergroup differences were non-significant. Healing index values of the PRF group during the first two weeks were statistically superior to those of the CTG control. One patient from the PRF group and seven from the CTG group experienced severe pain. Also, all of the patients in the control group reported some discomfort. Pain intensity was statistically superior in CTG during the first week. | [21] |
| Maxillary Sinus Augmentation (Graft) | 10/11 | (1) PRF + Bio-Oss® (2) Bio-Oss® | 6 | Healing was uneventful for all patients. Both groups exhibited an adequate amount and density of radiographic mineralized tissue plus a similar composition, distribution, and inflammation of histological structures. Intergroup differences were non-significant. The percentage of newly formed bone was about 1.4 times greater in the PRF group (18.35 ± 5.62% vs. 12.95 ± 5.33% of control). The percentage of residual bone substitute material was about 1.5 times greater in the control group (28.54 ± 12.01% vs. 19.16 ± 6.89% of LPRF). The bone-to-bone substitute contact was 21.45 ± 14.57% and 18.75 ± 5.39% in the PRF and the control group, respectively. Intergroup differences were non-significant. | [22] |
| Maxillary Sinus Augmentation (Membrane) | 6/12 | (1) PRF (2) Bio-Gide® | 5 | Wound healing was uneventful for all patients. No soft tissue in-growths were observed in both groups. Surfaces seemed homogenous with visible bone-substitute material embedded into newly-formed bone. The average amount of vital bone and bone substitute were 17.0% and 15.9% in the PRF group. Control group had 17.2% and 17.3%. No intergroup comparisons were carried out. | [23] |
| Alveolar Socket Preservation | 20/40 | (1) PRF (2) Empty (blood clot) | 3 | Soft tissue healing was significantly better in the PRF group vs. the controls (Laundry, Turnbell and Howley Soft Tissue Healing Index). Early bone formation/maturation was noticed for the experimental sites vs. controls. Differences were significant only at eight weeks post-extraction and favored the PRF group. Higher bone density was noticed in the PRF group vs. controls. Intergroup differences were non-significant. Mean post-surgical pain (measured by Visual Analogue Scale (VAS) score) was reduced in the PRF group vs. non PRF controls at day 1. By day 7, no intergroup differences were noticed. | [24] |
| Recommended Preparation Protocol for Clinically-fit L-PRF (Clots, Plugs, Blocks and Membranes) | |
|---|---|
| Collect 5–9 mL whole venous blood sample into 2–3 sterile 6 mL glass-coated plastic vacutainer tubes (without anti-coagulant -clot formation). |  |
| Centrifuge immediately for 10–12 min at 2700–3000 rpm (revolutions per minute) using any high-quality table-top centrifuge. | |
| Fibrinogen → Fibrin. Typically, 3 distinct compartments should be evident in each tube. UPPER Portion: straw-colored acellular plasma (PPP); MIDDLE Portion: yellowish fibrin clot (FC); and LOWER Fraction: red-colored corpuscles of red blood cells (RBCs). | |
| Quickly remove the upper layer to reveal and collect the middle portion; around 2 mm below the lower dividing line. Timing is critical to obtain bioactive L-PRFs charged with serum and platelets. This clot can then be used directly, either (a) as filling material; (b) mixed with bone grafting materials(s) – plugs and blocks; and/or (c) compressed (using the surgical box to prevent damage and to collect fibrin surgical glue in reservoir) into a strong and resilient clinically-usable membrane. For injectable PRF or iPRF (a more liquid or flowable formulation), centrigue tube again for 3–4 more minutes and collect top 1 mL layer using a syringe suitable for immediate injection into the intended application site. Mixing with other biomaterials is feasible as well. Note: slower centrifugation (less than 1500 rpm)for less time period (around 6–8 min) will result in a preparation with higher white blood cell count, commonly termed Advanced PRF or A-PRF suitable for defects requiring more vascularization (5 min needed to induce fibrin clot formation). | |
© 2018 by the authors. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zumarán, C.C.; Parra, M.V.; Olate, S.A.; Fernández, E.G.; Muñoz, F.T.; Haidar, Z.S. The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials 2018, 11, 1293. https://doi.org/10.3390/ma11081293
Zumarán CC, Parra MV, Olate SA, Fernández EG, Muñoz FT, Haidar ZS. The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials. 2018; 11(8):1293. https://doi.org/10.3390/ma11081293
Chicago/Turabian StyleZumarán, Consuelo C., Marcelo V. Parra, Sergio A. Olate, Eduardo G. Fernández, Francisco T. Muñoz, and Ziyad S. Haidar. 2018. "The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration" Materials 11, no. 8: 1293. https://doi.org/10.3390/ma11081293
APA StyleZumarán, C. C., Parra, M. V., Olate, S. A., Fernández, E. G., Muñoz, F. T., & Haidar, Z. S. (2018). The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials, 11(8), 1293. https://doi.org/10.3390/ma11081293