Corrosion Behavior and Mechanism of Basalt Fibers in Sodium Hydroxide Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Fiber Treatment
2.3. Measurement and Characterization
3. Results and Discussion
3.1. Mass Loss
3.2. Tensile Behavior of Basalt Fibers
3.3. SEM Image Analysis
4. Further Discussions
4.1. Corrosion Process of Basalt Fibers in NaOH Solution
4.2. Variation Trend of Tensile Strength of Basalt Fibers with Increase of Corrosion Time
4.3. Variation Trend of Tensile Strength of Basalt Fibers with Increase of Concentration
5. Conclusions
- The hydroxyl ions disrupt the –Si–O–Si– and –Si–O–Al– bonds, leading to the formation of insoluble hydroxides with high calcium and iron content on the fiber surface. With continuation of the hydration reaction, a thin hydrated layer (corrosion shell) covering the whole fiber surface was formed. The corrosion shell caused a significant increase in the strength and elongation at break of basalt fibers.
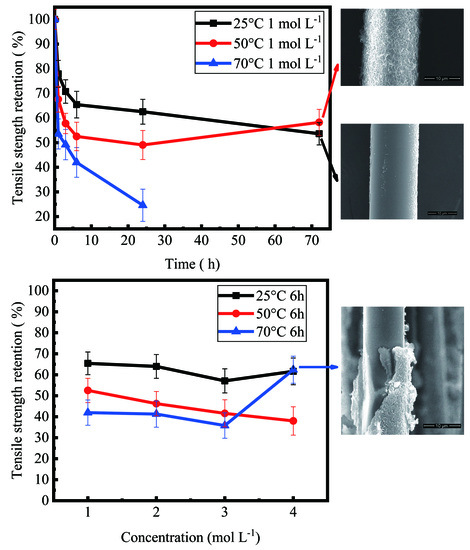
- For different temperatures, the degraded fibers showed different variation trends of tensile strength, as the corrosion time increased. When the basalt fibers were immersed in 1 mol/L NaOH solution at temperature of 25 °C, after 1 h, 3 h, 6 h, 1 day and 3 days, their retention ratios of strength were 77.9%, 70.7%, 65.4%, 62.5%, 53.6%, respectively. When the temperature rose to 50 °C, their retention ratios of strength were 67.6%, 57.8%, 52.5%, 49.0%, 58.2%, respectively.
- A higher temperature accelerated the corrosion rate of basalt fibers in NaOH solution. Due to the leaching of silicon, aluminum and potassium ions, the mass loss ratio of basalt fibers increased with the increase of temperature. For basalt fibers immersed in 1 mol/L NaOH solution for 3 days, the mass loss ratios were 2.4%, 16.6% and 33.8% when the temperature were 25 °C, 50 °C and 70 °C, respectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayrilmis, N.; Jarusombuti, S.; Fueangvivat, V.; Bauchongkol, P.; White, R.H. Coir fiber reinforced polypropylene composite panel for automotive interior applications. Fibers Polym. 2011, 12, 919–926. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, H.; Sun, M.; Wang, F.; Chen, B. Influence of embedding sma fibres and sma fibre surface modification on the mechanical performance of bfrp composite laminates. Materials 2018, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, W.; Gong, Z.; Li, W. Flexural progressive failure of carbon/glass interlayer and intralayer hybrid composites. Materials 2018, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Tran, H.D.; Hoang, V.T.; Do, V.T.; Chun, D.M.; Yum, Y.J. Effect of multiwalled carbon nanotubes on the mechanical properties of carbon fiber-reinforced polyamide-6/polypropylene composites for lightweight automotive parts. Materials 2018, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A review on basalt fibre and its composites. Compos. Part B 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Morozov, N.N.; Bakunov, V.S.; Morozov, E.N.; Aslanova, L.G.; Granovskii, P.A.; Prokshin, V.V.; Zemlyanitsyn, A.A. Materials based on basalts from the european north of russia. Glass Ceram. 2001, 58, 100–104. [Google Scholar] [CrossRef]
- Fiore, V.; Di Bella, G.; Valenza, A. Glass–basalt/epoxy hybrid composites for marine applications. Mater. Des. 2011, 32, 2091–2099. [Google Scholar] [CrossRef]
- Nasir, V.; Karimipour, H.; Taheri-Behrooz, F.; Shokrieh, M.M. Corrosion behaviour and crack formation mechanism of basalt fibre in sulphuric acid. Corros. Sci. 2012, 64, 1–7. [Google Scholar] [CrossRef]
- Hao, L.C.; Yu, W.D. Evaluation of thermal protective performance of basalt fiber nonwoven fabrics. J. Therm. Anal. Calorim. 2010, 100, 551–555. [Google Scholar] [CrossRef]
- Jang, K.S. Mechanics and rheology of basalt fiber-reinforced polycarbonate composites. Polymer 2018, 147, 133–141. [Google Scholar] [CrossRef]
- Choi, J.I.; Lee, B.Y. Bonding properties of basalt fiber and strength reduction according to fiber orientation. Materials 2015, 8, 6719–6727. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Gu, C.; Liu, J.; Liu, Y.; Li, M.; Lu, Y. Enhancement of the mechanical properties of basalt fiber-wood-plastic composites via maleic anhydride grafted high-density polyethylene (mape) addition. Materials 2013, 6, 2483–2496. [Google Scholar] [CrossRef] [PubMed]
- Dhand, V.; Mittal, G.; Rhee, K.Y.; Park, S.J.; Hui, D. A short review on basalt fiber reinforced polymer composites. Compos. Part B 2015, 73, 166–180. [Google Scholar] [CrossRef]
- Velpari, V.; Ramachandran, B.E.; Bhaskaran, T.A.; Pai, B.C.; Balasubramanian, N. Alkali resistance of fibres in cement. J. Mater. Sci. 1980, 15, 1579–1584. [Google Scholar] [CrossRef]
- Rabinovich, F.N.; Zueva, V.N.; Makeeva, L.V. Stability of basalt fibers in a medium of hydrating cement. Glass Ceram. 2001, 58, 431–434. [Google Scholar] [CrossRef]
- Sim, J.; Park, C.; Moon, D.Y. Characteristics of basalt fiber as a strengthening material for concrete structures. Compos. Part B 2005, 36, 504–512. [Google Scholar] [CrossRef]
- Zhang, N.; Zhong, Z.L.; Liu, H.W.; Zhang, H.J.; Lv, C. The research and development of continuous basalt fiber/polyester woven filter cloth. Adv. Mater. Res. 2011, 332–334, 973–976. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Li, Y.; Li, M.; Sun, Z. Chemical durability and mechanical properties of alkali-proof basalt fiber and its reinforced epoxy composites. J. Reinf. Plast. Compos. 2008, 27, 393–407. [Google Scholar] [CrossRef]
- Lipatov, Y.V.; Gutnikov, S.I.; Manylov, M.S.; Zhukovskaya, E.S.; Lazoryak, B.I. High alkali-resistant basalt fiber for reinforcing concrete. Mater. Des. 2015, 73, 60–66. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Wu, Z.S.; Dong, Z.Q.; Zhang, G.C. Durability of basalt fibers and composites in corrosive environments. J. Compos. Mater. 2015, 49, 873–887. [Google Scholar] [CrossRef]
- Ying, S.; Zhou, X. Chemical and thermal resistance of basalt fiber in inclement environments. J. Wuhan Univ. Technol. 2013, 28, 560–565. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.; Song, S. Tensile behavior contrast of basalt and glass fibers after chemical treatment. Mater. Des. 2010, 31, 4244–4250. [Google Scholar] [CrossRef]
- Rybin, V.A.; Utkin, А.V.; Baklanova, N.I. Corrosion of uncoated and oxide-coated basalt fibre in different alkaline media. Corros. Sci. 2016, 102, 503–509. [Google Scholar] [CrossRef]
- Altalmas, A.; El Refai, A.; Abed, F. Bond degradation of basalt fiber-reinforced polymer (bfrp) bars exposed to accelerated aging conditions. Constr. Build. Mater. 2015, 81, 162–171. [Google Scholar] [CrossRef]
- Ramachandran, B.E.; Velpari, V.; Balasubramanian, N. Chemical durability studies on basalt fibres. J. Mater. Sci. 1981, 16, 3393–3397. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, T.; Pan, Q.; Cheng, J.; Zhao, S. Corrosion behaviour and mechanism of basalt fibres in acidic and alkaline environments. Corros. Sci. 2016, 110, 15–22. [Google Scholar] [CrossRef]
- Rybin, V.A.; Utkin, А.V.; Baklanova, N.I. Alkali resistance, microstructural and mechanical performance of zirconia-coated basalt fibers. Cem. Concr. Res. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Aiello, M.A.; Ombres, L. Environmental effects on the mechanical properties of glass-frp and aramid-frp rebars. Mech. Compos. Mater. 2000, 36, 395–398. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, M.; Wang, Y.; Wu, G.; Wu, Z. Tensile behaviors of ECR-glass and high strength glass fibers after naoh treatment. Ceram. Int. 2013, 39, 9173–9178. [Google Scholar] [CrossRef]
- Lee, J.J.; Song, J.; Kim, H. Chemical stability of basalt fiber in alkaline solution. Fibers Polym. 2014, 15, 2329–2334. [Google Scholar] [CrossRef]
- Scheffler, C.; Förster, T.; Mäder, E.; Heinrich, G.; Hempel, S.; Mechtcherine, V. Aging of alkali-resistant glass and basalt fibers in alkaline solutions: Evaluation of the failure stress by weibull distribution function. J. Non-Cryst. Solids 2009, 355, 2588–2595. [Google Scholar] [CrossRef]
- Jantzen, C.; Brown, K.; Pickett, J.B. Durable glass for thousands of years. Int. J. Appl. Glass Sci. 2010, 1, 38–62. [Google Scholar] [CrossRef]
- Paul, A. Chemical durability of glasses; a thermodynamic approach. J. Mater. Sci. 1977, 12, 2246–2268. [Google Scholar] [CrossRef]
- Scholze, H.; Helmreich, D.; Bakardjiev, I. Investigations of the behaviour of soda-lime-silica glasses in dilute acids. Glastech. Ber. 1975, 48, 237–247. [Google Scholar]
- Liu, M.Y.; Zhu, H.G.; Siddiqui, N.A.; Leung, C.K.Y.; Kim, J.K. Glass fibers with clay nanocomposite coating: Improved barrier resistance in alkaline environment. Compos. Part A 2011, 42, 2051–2059. [Google Scholar] [CrossRef]












| Component Percentage | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | Na2O | TiO2 | MnO | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Desized basalt fibers (wt.%) | 47.0 | 16.0 | 15.1 | 9.2 | 4.1 | 3.5 | 1.4 | 0.2 | 3.1 | 0.3 |
| Parameter | Desized Basalt Fibers |
|---|---|
| Tensile strength (MPa) | 2300 ± 200 |
| Tensile modulus (GPa) | 62.6 ± 3 |
| Elongation at break (%) | 3.7 ± 0.2 |
| Component Percentage | SiO2 | Al2O3 | K2O | P2O5 | Fe2O3 | CaO | MgO | TiO2 | Na2O | MnO |
|---|---|---|---|---|---|---|---|---|---|---|
| Desized basalt fibers (wt.%) | 47.0 | 15.1 | 3.1 | 0.3 | 16.0 | 9.2 | 4.1 | 1.4 | 3.5 | 0.2 |
| Degraded basalt fibers (wt.%) | 33.9 | 8.1 | 2.0 | 0.1 | 25.0 | 15.5 | 8.8 | 2.4 | 3.7 | 0.5 |
| Percentage change (%) | +13.1 | +7.0 | +1.1 | +0.2 | −9.0 | −6.3 | −4.7 | −1.0 | −0.2 | −0.3 |
| Measured Point | Element Content (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Na | Mg | Al | Si | P | K | Ca | Ti | Mn | Fe | |
| Point A | 38.9 | 2.7 | 3.4 | 9.2 | 30.6 | 0.5 | 2.5 | 4.9 | 0.7 | 0.1 | 6.6 |
| Point B | 42.2 | 1.8 | 5.0 | 5.7 | 21.4 | 0.5 | 1.5 | 10.0 | 1.4 | 0.3 | 10.4 |
| Point C | 49.8 | 2.8 | 3.4 | 7.8 | 24.8 | 0.5 | 1.5 | 3.8 | 0.4 | 0.0 | 5.2 |
| Point D | 50.7 | 1.8 | 2.9 | 2.3 | 14.2 | 0.3 | 0.7 | 12.6 | 1.6 | 0.3 | 12.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Jiang, H.; Zhang, X.; Li, G.; Cui, J. Corrosion Behavior and Mechanism of Basalt Fibers in Sodium Hydroxide Solution. Materials 2018, 11, 1381. https://doi.org/10.3390/ma11081381
Tang C, Jiang H, Zhang X, Li G, Cui J. Corrosion Behavior and Mechanism of Basalt Fibers in Sodium Hydroxide Solution. Materials. 2018; 11(8):1381. https://doi.org/10.3390/ma11081381
Chicago/Turabian StyleTang, Chunhong, Hao Jiang, Xu Zhang, Guangyao Li, and Junjia Cui. 2018. "Corrosion Behavior and Mechanism of Basalt Fibers in Sodium Hydroxide Solution" Materials 11, no. 8: 1381. https://doi.org/10.3390/ma11081381
APA StyleTang, C., Jiang, H., Zhang, X., Li, G., & Cui, J. (2018). Corrosion Behavior and Mechanism of Basalt Fibers in Sodium Hydroxide Solution. Materials, 11(8), 1381. https://doi.org/10.3390/ma11081381