Graphene Quantum Dots Decorated Al-Doped ZnS for Improved Photoelectric Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of GQDs
2.3. Synthesis of ZnS, Al-ZnS, Al-ZnS/GQDs Composites
2.4. Characterization
2.5. Photocurrent Measurements
3. Results and Discussion
3.1. XRD Analysis
3.2. XPS Analysis
3.3. TEM Analysis
3.4. UV-vis Absorption Spectra
3.5. PL Spectra
3.6. FTIR Transmission Spectra
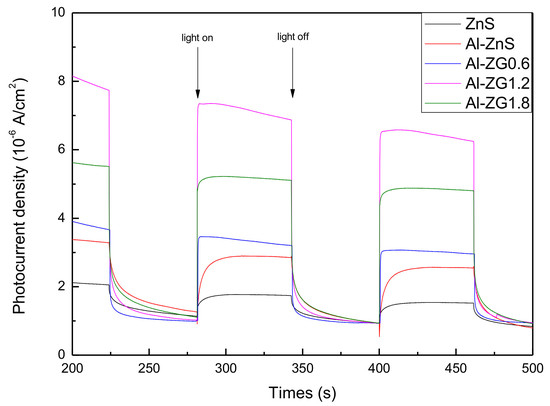
3.7. Photocurrent-Time Tests
3.8. Electrochemical Impedance Spectra
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ummartyotin, S.; Infahsaeng, Y. A comprehensive review on ZnS: From synthesis to an approach on solar cell. Renew. Sust. Energ. Rev. 2016, 55, 17–24. [Google Scholar] [CrossRef]
- Ghezali, K.; Mentar, L.; Boudine, B.; Azizi, A. Electrochemical deposition of ZnS thin films and their structural, morphological and optical properties. J. Electroanal. Chem. 2017, 794, 212–220. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, Z.; Duo, S.W.; Zhong, R.F.; Liu, T.Z. Structural, optical, photocurrent and mechanism-induced photocatalytic properties of surface-modified ZnS thin films by chemical bath deposition. J. Mater. Sci. Mater. Electron. 2017, 28, 28–42. [Google Scholar] [CrossRef]
- Sabaghi, V.; Davar, F.; Fereshteh, Z. ZnS nanoparticles prepared via simple reflux and hydrothermal method: Optical and photocatalytic properties. Ceram. Int. 2018, 44, 7545–7556. [Google Scholar] [CrossRef]
- Tuan, N.T.; Trung, D.Q.; Quang, N.V.; Hunh, N.D.; Khoi, N.T.; Huy, P.T.; Smet, P.F.; Meerte, K.W.; Poelman, D. Excitation energy dependence of the life time of orange emission from Mn-doped ZnS nanocrystals. J. Lumin. 2018, 199, 39–44. [Google Scholar] [CrossRef]
- Axelevitch, A.; Apter, B. Preparation and study of doped ZnS thin films. Microelectron. Eng. 2017, 170, 39–43. [Google Scholar] [CrossRef]
- Tian, X.Y.; Chen, Z.J.; Wen, J.; Du, Y.; Hu, J.L.; Wang, S.M.; Peng, H.X.; Li, J.; Peng, Y.X. Bio-inspired synthesis and optical properties of Dy3+-doped ZnS nanoparticles. J. Ceram. Process Res. 2017, 18, 116–121. [Google Scholar]
- Sotillo, B.; Fernández, P.; Piqueras, J. Growth by thermal evaporation of Al doped ZnS elongated micro- and nanostructures and their cathodoluminescence properties. J. Alloys Compd. 2014, 603, 57–64. [Google Scholar] [CrossRef]
- Jiang, P.; Jie, J.S.; Yu, Y.Q.; Wang, Z.; Xie, C.; Zhang, X.W.; Wu, C.Y.; Wang, L.; Zhu, Z.F.; Luo, L.B. Aluminium-doped n-type ZnS nanowires as high-performance UV and humidity sensors. J. Mater. Chem. 2012, 22, 6856–6861. [Google Scholar] [CrossRef]
- Amaranatha, R.D.; Liu, C.L.; Vijayalakshmi, R.P.; Reddy, B.K. Effect of Al doping on the structural, optical and photoluminescence properties of ZnS nanoparticles. J. Alloys Compd. 2014, 582, 257–264. [Google Scholar] [CrossRef]
- Gawai, U.P.; Deshpande, U.P.; Dole, B.N. A study on the synthesis, longitudinal optical phonon-plasmon coupling and electronic structure of Al doped ZnS nanorods. RSC Adv. 2017, 7, 12382–12390. [Google Scholar] [CrossRef]
- Bitri, N.; Ben, B.K.; Ly, I.; Bouzouita, H.; Abaab, M. Studies of physical properties of the Al doped ZnS thin films prepared by Spray. J. Mater. Sci. Mater. Electron. 2017, 28, 734–744. [Google Scholar] [CrossRef]
- Ma, M.J.F.; Hu, X.Y.; Zhang, C.B.; Deng, C.Y.; Wang, X. The optimum parameters to synthesize bright and stable graphene quantum dots by hydrothermal method. J. Mater. Sci. Mater. Electron. 2017, 28, 6493–6497. [Google Scholar] [CrossRef]
- Liu, T.; Yu, K.; Gao, L.N.; Chen, H.; Wang, N.; Hao, L.H.; Li, T.X.; He, H.C.; Guo, Z.H. A graphene quantum dot decorated SrRuO3 mesoporous film as an efficient counter electrode for high-performance dye-sensitized solar cells. J. Mater. Chem. A. 2017, 5, 17848–17855. [Google Scholar] [CrossRef]
- Liu, J.Y.; Xu, H.; Xu, Y.G.; Song, Y.H.; Lian, J.B.; Zhao, Y.; Wang, L.; Huang, L.Y.; Ji, H.Y.; Li, H.M. Graphene quantum dots modified mesoporous graphite carbon nitride with significant enhancement of photocatalytic activity. Appl. Catal. B 2017, 207, 429–437. [Google Scholar] [CrossRef]
- Majumder, T.; Dhar, S.; Debnath, K.; Mondal, S.P. Role of S, N co-doped graphene quantum dots as a green photosensitizer with Ag-doped ZnO nanorods for improved electrochemical solar energy conversion. Mater. Res. Bull. 2017, 93, 214–222. [Google Scholar] [CrossRef]
- Yan, M.; Hua, Y.Q.; Zhu, F.F.; Gu, W.; Jiang, J.H.; Shen, H.Q.; Shi, W.D. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6p-n junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B 2017, 202, 518–527. [Google Scholar] [CrossRef]
- Ham, S.; Kim, Y.; Park, M.J.; Hong, B.H.; Jang, D. Graphene quantum dots-decorated ZnS nanobelts with highly efficient photocatalytic performances. RSC Adv. 2016, 6, 24115–24120. [Google Scholar] [CrossRef]
- Pan, D.Y.; Jiao, J.K.; Li, Z.; Guo, Y.T.; Feng, C.Q.; Liu, Y.; Wang, L.; Wu, M.H. Efficient separation of electron−hole pairs in graphene quantum dots by TiO2 heterojunctions for dye degradation. ACS Sustainable Chem. Eng. 2015, 3, 2405–2413. [Google Scholar] [CrossRef]
- Khan, S.; Narula, A.K. Synthesis of the ternary photocatalyst based on ZnO sensitized graphene quantum dot reinforced with conducting polymer exhibiting photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 6337–6349. [Google Scholar] [CrossRef]
- Lei, Y.G.; Yang, C.; Hou, J.H.; Wang, F.; Min, S.X.; Ma, X.H.; Jin, Z.L.; Xu, J.; Lu, G.X.; Huang, K. Strongly coupled CdS/graphene quantum dots nanohybrids for highly efficient photocatalytic hydrogen evolution: Unraveling the essential roles of graphene quantum dots. Appl. Catal. B 2017, 216, 59–69. [Google Scholar] [CrossRef]
- Shi, J.J.; Meng, L.X.; Yang, P. Ultrasensitive determination of 2, 4, 6-trinitrotoluene by exploiting the strongly enhanced electrochemiluminescence of an assembly between CdSe and graphene quantum dots and its quenching by TNT. Microchim. Acta 2017, 184, 73–80. [Google Scholar] [CrossRef]
- Xie, J.S.; Huang, K.; Yu, X.G.; Yang, Z.R.; Xiao, K.; Qiang, Y.P.; Zhu, X.D.; Xu, L.B.; Wang, P.; Cui, C.; et al. Enhanced electronic properties of SnO2 via electron transfer from graphene quantum dots for efficient perovskite solar cells. ACS Nano. 2017, 11, 9176–9182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fang, C.Y.; Bing, X.; Lei, Y. Graphene quantum dots-ZnS nanocomposites with improved photoelectric performances. Materials 2018, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Z.; Liu, M.X.; Shao, X.; Yang, S.Y. ZnS/graphene hybrid nanomaterials: Synthesis, characterization, and enhanced electrochemical performances. J. Mater. Sci. Mater. Electron. 2015, 26, 6495. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.W.; Xu, Y.L.; Zhang, Y.Y.; Wu, J.L. Preparation and characterisation of graphene/ZnS nanocomposites via a surfactant-free method. J. Exp. Nanosci. 2014, 9, 415–420. [Google Scholar] [CrossRef]
- Shanmugam, M.; Alsalme, A.; Alghamdi, A.; Jayavel, R. Enhanced Photocatalytic Performance of the Graphene-V2O5 Nanocomposite in the Degradation of Methylene Blue Dye under Direct Sunlight. ACS Appl. Mater. Interfaces. 2015, 7, 14905–14911. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.L.; Liu, W.W.; Tan, P.F.; Zhu, A.Q.; Liu, Y.; Xiong, X.; Pan, J. Insights into the synergy effect of anisotropic {001} and {230} facets of BaTiO3 nanocubes sensitized with CdSe quantum dots for photocatalytic water reduction. Appl. Catal. B. 2018, 227, 1–12. [Google Scholar] [CrossRef]
- Wen, L.; Kumar, M.; Cho, H.J.; Leksakul, K.; Han, J.G. Low-bandgap, highly c-axis-oriented Al-doped ZnO thin films. J. Phys. D Appl. Phys. 2017, 50, 185206. [Google Scholar] [CrossRef]
- Kumar, M.; Wen, L.; Han, J.G. Plasma diagnostic of cup-like magnetron source for transparent conductive oxide thin films. Vacuum 2017, 146, 517–523. [Google Scholar] [CrossRef]
- Kumar, M.; Wen, L.; Sahu, B.B.; Han, J.G. Simultaneous enhancement of carrier mobility and concentration via tailoring of Al-chemical states in Al-ZnO thin films. Appl. Phys. Lett. 2015, 106, 241903. [Google Scholar] [CrossRef]








| Sample | Atomic% (at %) | |||
|---|---|---|---|---|
| Zn | S | Al | C | |
| ZnS | 50.05 | 49.95 | 0 | 0 |
| Al-ZnS | 49.37 | 46.05 | 2.28 | 0 |
| Al-ZG | 46.28 | 43.15 | 2.48 | 7.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Lei, Y.; Zhao, L.; Jiang, Z.; Ouyang, Z. Graphene Quantum Dots Decorated Al-Doped ZnS for Improved Photoelectric Performance. Materials 2018, 11, 1452. https://doi.org/10.3390/ma11081452
Zhang Z, Lei Y, Zhao L, Jiang Z, Ouyang Z. Graphene Quantum Dots Decorated Al-Doped ZnS for Improved Photoelectric Performance. Materials. 2018; 11(8):1452. https://doi.org/10.3390/ma11081452
Chicago/Turabian StyleZhang, Zheng, Yun Lei, Liyang Zhao, Zicong Jiang, and Zhong Ouyang. 2018. "Graphene Quantum Dots Decorated Al-Doped ZnS for Improved Photoelectric Performance" Materials 11, no. 8: 1452. https://doi.org/10.3390/ma11081452
APA StyleZhang, Z., Lei, Y., Zhao, L., Jiang, Z., & Ouyang, Z. (2018). Graphene Quantum Dots Decorated Al-Doped ZnS for Improved Photoelectric Performance. Materials, 11(8), 1452. https://doi.org/10.3390/ma11081452