TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2-KH550
2.3. Characterization of TiO2-KH550
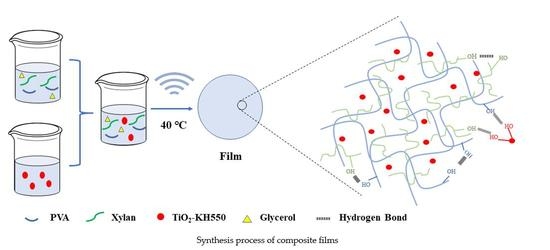
2.4. Preparation of TiO2-KH550 Reinforced PVA/xylan Composite Films
2.5. Characterization of Composite Films
3. Results and Discussion
3.1. Characterizations of TiO2-KH550
3.2. Characterizations of Films
3.2.1. Mechanical Properties
3.2.2. Water Vapor Permeability, Solubility, and Oxygen Permeability
3.2.3. Moisture Absorption
3.2.4. Thermal Stability
3.2.5. Ultraviolet Light Shielding Performance
3.2.6. XRD Analysis
3.2.7. SEM Analysis
3.2.8. AFM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grondahl, M.; Eriksson, L.; Gatenholm, P. Material properties of plasticized hardwood xylans for potential application as oxygen barrier films. Biomacromolecules 2004, 5, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.M.L.; Plackett, D. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Albertsson, A.C.; Lindblad, M.S.; Sjoberg, J. Oxygen barrier materials from renewable sources: Material properties of softwood hemicellulose-based films. J. App. Polym. Sci. 2006, 100, 2985–2991. [Google Scholar] [CrossRef]
- Gabrielii, I.; Gatenholm, P.; Glasser, W.G.; Jain, R.K.; Kenne, L. Separation, characterization and hydrogel-formation of hemicellulose from aspen wood. Carbohydr. Polym. 2000, 43, 367–374. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Tenkanen, M. Sustainable food-packaging materials based on future biorefinery products: Xylans and mannans. Trends Food. Sci. Technol. 2012, 28, 90–102. [Google Scholar] [CrossRef]
- Priya, B.; Gupta, V.K.; Pathania, D.; Singha, A.S. Synthesis, characterization and antibacterial activity of biodegradable starch/PVA composite films reinforced with cellulosic fiber. Carbohydr. Polym. 2014, 109, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.Z.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ren, J.L.; Kong, W.Q.; Gao, C.D.; Liu, C.F.; Peng, F.; Sun, R.C. Influence of urea and glycerol on functional properties of biodegradable PVA/xylan composite films. Cellulose 2014, 21, 495–505. [Google Scholar] [CrossRef]
- Ren, J.L.; Wang, S.Y.; Gao, C.D.; Chen, X.F.; Li, W.Y.; Peng, F. TiO2-containing PVA/xylan composite films with enhanced mechanical properties, high hydrophobicity and UV shielding performance. Cellulose 2015, 22, 593–602. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ren, J.L.; Li, W.Y.; Sun, R.C.; Liu, S.J. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.D.; Ren, J.L.; Wang, S.Y.; Sun, R.C.; Zhao, L.H. Preparation of Polyvinyl Alcohol/Xylan Blending Films with 1,2,3,4-Butane Tetracarboxylic Acid as a New Plasticizer. J. Nanomater. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Gomez-Romero, G. Hybrid organic-inorganic materials-In search of synergic activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Kovaevic, V.; Vrsaljko, D.; Blagojevic, S.L.; Leskovac, M. Adhesion parameters at the interface in nanoparticulate filled polymer systems. Polym. Eng. Sci. 2008, 48, 1994–2002. [Google Scholar] [CrossRef]
- Chen, X.B.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Serrano, C.; Ferrer, M.; Lunsdorf, H.; Bielecki, P.; Cerrada, M.A.L.; Fernandez-Garcia, M.; Fernandez-Garcia, M. High-performance dual-action polymer-TiO2 nanocomposite films via melting processing. Nano Lett. 2007, 7, 2529–2534. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.H.; Lee, S.C.; Yu, J.C. Photocatalyst TiO2 supported on glass fiber for indoor air purification: Effect of NO on the photodegradation of CO and NO2. J. Photoch. Photobiol. A 2003, 156, 171–177. [Google Scholar] [CrossRef]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photoch. Photobiol. C 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Byrne, J.A.; Eggins, B.R.; Brown, N.M.D.; Mckinney, B.; Rouse, M. Immobilisation of TiO2 powder for the treatment of polluted water. Appl. Catal. B. Environ. 1998, 17, 25–36. [Google Scholar] [CrossRef]
- Cozmuta, A.N.; Peter, A.; Cozmuta, L.M.; Nicula, C.; Crisan, L.; Baia, L.; Turila, A. Active packaging system based on Ag/TiO2 nanocomposite used for extending the shelf life of bread, chemical and microbiological investigations. Packag. Technol. Sci. 2015, 28, 271–284. [Google Scholar] [CrossRef]
- Fang, D.L.; Yang, W.J.; Kimatu, B.M.; Mariga, A.M.; Zhao, L.Y.; An, X.X.; Hu, Q.H. Effect of nanocomposite-based packaging on storage stability of mushrooms (Flammulina velutipes). Innov. Food Sci. Emerg. 2016, 33, 489–497. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Al-Harthi, M.A.; De, S.K. Reinforcement of starch/polyvinyl alcohol blend using nano-titanium dioxide. J. Compos. Mater. 2012, 46, 3181–3187. [Google Scholar] [CrossRef]
- Lou, Y.H.; Liu, M.H.; Miao, X.W.; Zhang, L.; Wang, X.P. Improvement of the mechanical properties of nano-TiO2/poly(vinyl alcohol) composites by enhanced interaction between nanofiller and matrix. Polym. Compos. 2010, 31, 1184–1193. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Charan, S. Synthesis of nano-particles of anatase-TiO2 and preparation of its optically transparent film in PVA. Mater. Lett. 2007, 61, 4725–4730. [Google Scholar] [CrossRef]
- Aruna, S.T.; Anandan, C.; Grips, V.K.W. Effect of probe sonication and sodium hexametaphosphate on the microhardness and wear behavior of electrodeposited Ni-SiC composite coating. Appl. Surf. Sci. 2014, 301, 383–390. [Google Scholar] [CrossRef]
- Chang, G.; He, L.; Zheng, W.; Pan, A.Z.; Liu, J.; Li, Y.J.; Cao, R.J. Well-defined inorganic/organic nanocomposite by nano silica core-poly(methyl methacrylate/butylacrylate/trifluoroethyl methacrylate) shell. J. Colloid Interface Sci. 2013, 396, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Zhang, Q.Y.; Li, H.C.; Tang, Y.S.; Kong, J.; Dang, J. Study on preparation of SiO2/epoxy resin hybrid materials by means of sol-gel. Polym.-Plast. Technol. 2007, 46, 1129–1134. [Google Scholar] [CrossRef]
- Li, X.W.; Song, R.G.; Jiang, Y.; Wang, C.; Jiang, D. Surface modification of TiO2 nanoparticles and its effect on the properties of fluoropolymer/TiO2 nanocomposite coatings. Appl. Surf. Sci. 2013, 276, 761–768. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Kayserilioglu, B.S.; Stevels, W.M.; Mulder, W.J.; Akkas, N. Mechanical and biochemical characterisation of wheat gluten films as a function of pH and co-solvent. Starch-Starke 2001, 53, 381–386. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A. Characterization of TiO2 and TiO2-MnO oxides prepared by sol-gel method. Solid State Ionics 2001, 138, 227–232. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Q.; Xie, Y.T.; Chen, Q.; Ouyang, C.F.; Xu, Y.M.; Ji, X.T. Effect of SiO2 and TiO2 nanoparticle on the properties of phenyl silicone rubber. J. Appl. Polym. Sci. 2015, 132, 1–9. [Google Scholar] [CrossRef]
- Ukaji, E.; Furusawa, T.; Sato, M.; Suzuki, N. The effect of surface modification with silane coupling agent on suppressing the photo-catalytic activity of fine TiO2 particles as inorganic UV filter. Appl. Surf. Sci. 2007, 254, 563–569. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, N.M.G.G.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surf. A 2012, 413, 273–279. [Google Scholar] [CrossRef]
- Wang, S.Y. Influence of Additives on Properties of Poly Vinyl Alcohol/Xylan Composite Films; South China University of Technology: Guangzhou, China, 2014; pp. 1–84. [Google Scholar]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mater. Sci. Eng. C-Mater. 2015, 57, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xiong, H.G.; Chen, X.; Lin, S.; Tu, Y.H. Effects of nano-TiO2 on the performance of high-amylose starch based antibacterial films. J. Appl. Polym. Sci. 2015, 132, 1–7. [Google Scholar] [CrossRef]
- Shi, F.M.; Ma, Y.X.; Ma, J.; Wang, P.P.; Sun, W.X. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Membr. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Feng, X.X.; Zhang, L.L.; Chen, J.Y.; Guo, Y.H.; Zhang, H.P.; Jia, C.L. Preparation and characterization of novel nanocomposite films formed from silk fibroin and nano-TiO2. Int. J. Biol. Macromol. 2007, 40, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pourjafar, S.; Rahimpour, A.; Jahanshahi, M. Synthesis and characterization of PVA/PES thin film composite nanofiltration membrane modified with TiO2 nanoparticles for better performance and surface properties. J. Ind. Eng. Chem. 2012, 18, 1398–1405. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Mumjitha, M.; Purnasai, K.; Raj, V. Fabrication and characterization of poly (vinyl alcohol)-TiO2 nanocomposite films for orthopedic applications. J. Mech. Behav. Biomed. 2016, 63, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Macyk, W.; Szacilowski, K.; Stochel, G.; Buchalska, M.; Kuncewicz, J.; Labuz, P. Titanium(IV) complexes as direct TiO2 photosensitizers. Coord. Chem. Rev. 2010, 254, 2687–2701. [Google Scholar] [CrossRef]
- Li, Y.X.; Jiang, Y.F.; Liu, F.; Ren, F.Z.; Zhao, G.H.; Leng, X.J. Fabrication and characterization of TiO2/whey protein isolate nanocomposite film. Food Hydrocolloid 2011, 25, 1098–1104. [Google Scholar] [CrossRef]
- Chiang, P.C.; Whang, W.T. The synthesis and morphology characteristic study of BAO-ODPA polyimide/TiO2 nano hybrid films. Polymer 2003, 44, 2249–2254. [Google Scholar] [CrossRef]
- Mallakpour, S.; Barati, A. Efficient preparation of hybrid nanocomposite coatings based on poly(vinyl alcohol) and silane coupling agent modified TiO2 nanoparticles. Prog. Org. Coat. 2011, 71, 391–398. [Google Scholar] [CrossRef]
- Gao, C.D.; Ren, J.L.; Kong, W.Q.; Sun, R.C.; Chen, Q.F. Comparative study on temperature/pH sensitive xylan-based hydrogels: Their properties and drug controlled release. RSC Adv. 2015, 5, 90671–90681. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, K.; Tian, X.Y.; Hu, K.; Wang, R.X.; Liu, C.; Li, Y.; Cui, P. Double glass transitions and interfacial immobilized layer in in-situ-synthesized poly(vinyl alcohol)/silica nanocomposites. Macromolecules 2010, 43, 1076–1082. [Google Scholar] [CrossRef]
- Qin, A.W.; Li, X.; Zhao, X.Z.; Liu, D.P.; He, C.J. Engineering a highly hydrophilic PVDF membrane via binding TiO2 nanoparticles and a PVA layer onto a membrane surface. ACS Appl. Mater. Int. 2015, 7, 8427–8436. [Google Scholar] [CrossRef] [PubMed]











| Samples | WS (%) | WVP (10−11 g·s−1·m−1·Pa−1) | OP (cm3·m−2·24 h−1·0.1MPa−1) |
|---|---|---|---|
| KT0 | 32.38 ± 2.3 | 3.97 ± 0.8 | 6.823 |
| KT0.5 | 29.93 ± 1.8 | 3.25 ± 0.3 | 5.372 |
| KT1 | 27.72 ± 1.7 | 3.57 ± 0.6 | 4.092 |
| KT1.5 | 28.84 ± 2.3 | 2.75 ± 0.3 | 4.013 |
| KT2 | 29.78 ± 2.1 | 3.43 ± 0.5 | 6.138 |
| KT2.5 | 30.82 ± 2.6 | 3.61 ± 0.5 | 6.068 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, X.; Ren, J.; Zhang, C. TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties. Materials 2018, 11, 1589. https://doi.org/10.3390/ma11091589
Liu X, Chen X, Ren J, Zhang C. TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties. Materials. 2018; 11(9):1589. https://doi.org/10.3390/ma11091589
Chicago/Turabian StyleLiu, Xinxin, Xiaofeng Chen, Junli Ren, and Chunhui Zhang. 2018. "TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties" Materials 11, no. 9: 1589. https://doi.org/10.3390/ma11091589
APA StyleLiu, X., Chen, X., Ren, J., & Zhang, C. (2018). TiO2-KH550 Nanoparticle-Reinforced PVA/xylan Composite Films with Multifunctional Properties. Materials, 11(9), 1589. https://doi.org/10.3390/ma11091589