Nanomelanin Potentially Protects the Spleen from Radiotherapy-Associated Damage and Enhances Immunoactivity in Tumor-Bearing Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of Nanomelanin
2.3. Cells and Mice
2.4. Realtime-Polymerase Chain Reaction (PCR) Analysis
2.5. Tumor-Bearing Mice
2.6. Radiotherapy Treatment of Tumor-Bearing Mice
2.7. Flow Cytometry Analysis
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Nanomelanin from Melanin Powder
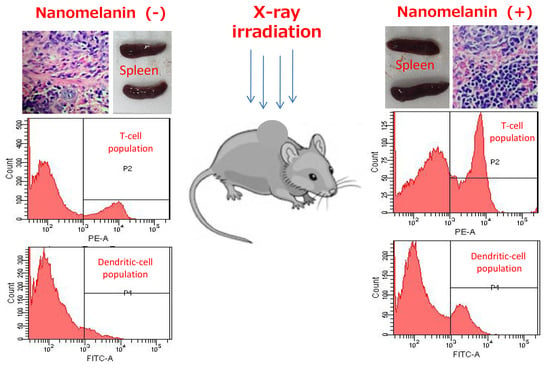
3.2. Nanomelanin Protected Mouse Spleens from X-radiation
3.3. Nanomelanin Enhanced Populations of Immune Cells in the Spleen of X-ray Irradiated-Mice
3.4. Nanomelanin Indirectly Activated Apoptotic Signaling in the Tumor Tissues of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.C.; West, C.M.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.; Burnet, N.G. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conklin, K.A. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef]
- Valerie, K.; Yacoub, A.; Hagan, M.P.; Curiel, D.T.; Fisher, P.B.; Grant, S.; Dent, P. Radiation-induced cell signaling: inside-out and outside-in. Mol. Cancer Ther. 2007, 6, 789–801. [Google Scholar] [CrossRef] [Green Version]
- Yamamori, T.; Yasui, H.; Yamazumi, M.; Wada, Y.; Nakamura, Y.; Nakamura, H.; Inanami, O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic. Biol. Med. 2012, 53, 260–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Hauer-Jensen, M. γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status. Int. J. Mol. Sci. 2016, 17, 663. [Google Scholar] [CrossRef]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Alsbeih, G. Appraisal of biochemical classes of radioprotectors: evidence, current status and guidelines for future development. 3 Biotech 2017, 7, 292. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Guo, B.; Zhu, B. Regulation of adaptive immune responses by guiding cell movements in the spleen. Front. Microbiol. 2015, 6, 645. [Google Scholar] [CrossRef] [Green Version]
- Rageh, M.M.; El-Gebaly, R.H.; Abou-Shady, H.; Amin, D.G. Melanin nanoparticles (MNPs) provide protection against whole-body ɣ-irradiation in mice via restoration of hematopoietic tissues. Mol. Cell. Biochem. 2015, 399, 59–69. [Google Scholar] [CrossRef]
- Kunwar, A.; Adhikary, B.; Jayakumar, S.; Barik, A.; Chattopadhyay, S.; Raghukumar, S.; Priyadarsini, K.I. Melanin, a promising radioprotector: mechanisms of actions in a mice model. Toxicol. Appl. Pharmacol. 2012, 264, 202–211. [Google Scholar] [CrossRef]
- Shang, N.; Figini, M.; Shangguan, J.; Wang, B.; Sun, C.; Pan, L.; Ma, Q.; Zhang, Z. Dendritic cells based immunotherapy. Am. J. Cancer Res. 2017, 7, 2091–2102. [Google Scholar]
- Sabado, R.L.; Bhardwaj, N. Directing dendritic cell immunotherapy towards successful cancer treatment. Immunotherapy 2010, 2, 37–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrest, B.A.; Eller, M.S.; Geller, A.C.; Yaar, M. The pathogenesis of melanoma induced by ultraviolet radiation. N. Engl. J. Med. 1999, 340, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Gerald, A.B.; Pathak, M.A.; Fitzpatrick, T.B. Racial differences in the fate of melanosomes in human epidermis. Nature 1969, 222, 1081–1082. [Google Scholar] [CrossRef]
- Garcia Borron, J.C.; Abdel-Malek, Z.; Jimenez, C.C. MC1R, the cAMP pathway, and the response to solar UV: extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef]
- Schweitzer, A.D.; Revskaya, E.; Chu, P.; Pazo, V.; Friedman, M.; Nosanchuk, J.D.; Cahill, S.; Frases, S.; Casadevall, A.; Dadachova, E. Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1494–1502. [Google Scholar] [CrossRef]
- Hu, D.N. Methodology for evaluation of melanin content and production of pigment cells in vitro. Photochem. Photobiol. 2008, 84, 645–649. [Google Scholar] [CrossRef]
- Cuong, A.M.; Le-Na, N.T.; Thang, P.N.; Diep, T.N.; Thuy, L.B.; Thanh, N.L.; Thang, N.D. Melanin-embedded materials effectively remove hexavalent chromium (CrVI) from aqueous solution. Environ Health Prev Med. 2018, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Simon, J.D. Metal-ion interactions and the structural organization of Sepia eumelanin. Pigment Cell Res. 2005, 18, 42–48. [Google Scholar] [CrossRef]
- Ho, Y.S.; Chiang, C.C.; Hsu, Y.C. Sorption kinetics for dye removal from aqueous solution using activated clay. Sep. Sci Technol. 2001, 36, 2473–2488. [Google Scholar] [CrossRef]
- Kaidbey, K.H.; Agin, P.P.; Sayre, R.M.; Kligman, A.M. Photoprotection by melanin—A comparison of black and Caucasian skin. J. Am. Acad. Dermatol. 1979, 1, 249–260. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Hayashi, M.; Hirai, R.; Ishihara, Y.; Horiguchi, N.; Endoh, D.; Okui, T. Combined effects of treatment with trientine, a copper-chelating agent, and x-irradiation on tumor growth in transplantation model of a murine fibrosarcoma. J. Vet. Med. Sci. 2007, 69, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Inano, H.; Onoda, M.; Murase, H.; Ikota, N.; Kagiya, T.V.; Anzai, K. Modification of mortality and tumorigenesis by tocopherol-mono-glucoside (TMG) administered after X irradiation in mice and rats. Radiat. Res. 2009, 172, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Anzai, K.; Ueno, M.; Matsumoto, K.; Ikota, N.; Takata, J. Gamma-tocopherol-N,N-dimethylglycine ester as a potent post-irradiation mitigator against whole body X-irradiation-induced bone marrow death in mice. J. Radiat. Res. 2014, 55, 67–74. [Google Scholar] [CrossRef]
- Abdullaev, S.; Minkabirova, G.; Karmanova, E.; Bruskov, V.; Gaziev, A. Metformin prolongs survival rate in mice and causes increased excretion of cell-free DNA in the urine of X-irradiated rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 831, 13–18. [Google Scholar] [CrossRef]
- Pozniak, M.A.; Christy, P.S.; Albertini, M.R.; Duffek, S.M.; Schiller, J.H. Interleukin-2-induced splenic enlargement. Cancer 1995, 75, 2737–2741. [Google Scholar] [CrossRef]
- Wu, X.; Wu, M.Y.; Jiang, M.; Zhi, Q.; Bian, X.; Xu, M.D.; Gong, F.R. TNF-α sensitizes chemotherapy and radiotherapy against breast cancer cells. Cancer Cell Int. 2017, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, R.; Ten-Hagen, T.L.; Eggermont, A.M. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Hey, Y.Y.; O’Neill, H.C. Murine spleen contains a diversity of myeloid and dendritic cells distinct in antigen presenting function. J. Cell Mol. Med. 2012, 16, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.H.; Metcalf, D.; van Nieuwenhuijze, A.; Wicks, I.; Wu, L.; O’Keeffe, M.; Shortman, K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 2006, 7, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Jung, S. Development and function of dendritic cell subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef]
- Griffiths, K.L.; Tan, J.K.; O’Neill, H.C. Characterization of the effect of LPS on dendritic cell subset discrimination in spleen. J. Cell Mol. Med. 2014, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Mathisen, S.; Schulthess, J.; Danne, C.; Hegazy, A.N.; Powrie, F. CD11c+ monocyte/macrophages promote chronic Helicobacter hepaticus-induced intestinal inflammation through the production of IL-23. Mucosal Immunol. 2016, 9, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, H.S.; Kim, M.A.; Ryu, H.; Jeong, H.J.; Lee, C.M. Thermohydrogel Containing Melanin for Photothermal Cancer Therapy. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.L.; Barrett, J.A.; Marquis, J.C.; Chen, J.; Hillier, S.M.; Maresca, K.P.; Boyd, M.; Gage, K.; Nimmagadda, S.; Kronauge, J.F.; et al. Preclinical evaluation of an 131I-labeled benzamide for targeted radiotherapy of metastatic melanoma. Cancer Res. 2010, 70, 4045–4053. [Google Scholar] [CrossRef]
- Dadachova, E.; Moadel, T.; Schweitzer, A.D.; Bryan, R.A.; Zhang, T.; Mints, L.; Revskaya, E.; Huang, X.; Ortiz, G.; Nosanchuk, J.S.; et al. Radiolabeled melanin-binding peptides are safe and effective in treatment of human pigmented melanomain a mouse model of disease. Cancer Biother. Radio. 2006, 21, 117–129. [Google Scholar]
- Wen, S.W.; Everitt, S.J.; Bedő, J.; Chabrot, M.; Ball, D.L.; Solomon, B.; MacManus, M.; Hicks, R.J.; Möller, A.; Leimgruber, A. Spleen Volume Variation in Patients with Locally Advanced Non Small Cell Lung Cancer Receiving Platinum-Based Chemo-Radiotherapy. PLoS ONE 2015, 10, e0142608. [Google Scholar] [CrossRef]
- Park, B.; Yee, C.; Lee, K.M. The effect of radiation on the immune response to cancers. Int. J. Mol. Sci. 2014, 15, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, D.; Stigbrand, T. Radiation-induced cell death mechanisms. Tumour Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Verheij, M.; Bartelink, H. Radiation-induced apoptosis. Cell Tissue Res. 2000, 301, 133–142. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Z.; Chen, F.; Zhang, W.; Song, S.; Song, S. Irradiation enhances dendritic cell potential antitumor activity by inducing tumor cell expressing TNF-α. Med. Oncol. 2017, 34, 44. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. T lymphocytes and normal tissue responses to radiation. Front. Oncol. 2012, 2, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiriva-Internati, M.; Grizzi, F.; Pinkston, J.; Morrow, K.J.; D’Cunha, N.; Frezza, E.E.; Muzzio, P.C.; Kast, W.M.; Cobos, E. Gamma-radiation upregulates MHC class I/II and ICAM-I molecules in multiple myeloma cell lines and primary tumors. In Vitro Cell. Dev. Biol. Anim. 2006, 42, 89–95. [Google Scholar]
- Ewald, J.A.; Desotelle, J.A.; Wilding, G.; Jarrard, D.F. Therapy-induced senescence in cancer. J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef] [PubMed]








| Gene Name | Sequence (5′ to 3′) |
|---|---|
| mouse TNF-α (F) | ATGAGCACAGAAAGCATGA |
| mouse TNF-α (R) | AGTAGACAGAAGAGCGTGGT |
| mouse IL-2 (F) | TTGTGCTCCTTGTCAACAGC |
| mouse IL-2 (R) | CTGGGGAGTTTCAGGTTCCT |
| mouse Caspase-3 (F) | CCTCAGAGAGACATTCATGG |
| mouse Caspase-3 (R) | GCAGTAGTCGCCTCTGAAGA |
| mouse Bax (F) | AGCAAACTGGTGCTCAAGGC |
| mouse Bax (R) | CCACAAAGATGGTCACTGTC |
| mouse GAPDH (F) | CCCATCACCATCTTCCAGGAGC |
| mouse GAPDH (R) | CCAGTGAGCTTCCCGTTCAGC |
| mouse β-actin (F) | CGGTTCCGATGCCCTGAGGCTCTT |
| mouse β-actin (R) | CGTCACACTTCATGATGGAATTGA |
| Parameters | Unit | NIL | NC | IR | IR + MEL |
|---|---|---|---|---|---|
| White Blood Cell (WBC) | [109/L] | 4.15 ± 0.98 | 3.8 ± 0.94 | 0.45 ±0.10 | 0.7 ± 0.22 |
| Red Blood Cell (RBC) | [1012/L] | 7.7 ± 0.52 | 6.68 ± 0.19 | 5.48 ± 1.09 | 5.21 ± 0.68 |
| Hemoglobin (HGB) | [g/dL] | 11 ± 0.61 | 10.63 ± 0.93 | 8.34 ± 0.65 | 7.77 ± 0.69 |
| Hemocratit (HCT) | [%] | 34.4 ± 2.69 | 33.1 ± 2.30 | 24.94 ± 2.05 | 23.1 ± 1.21 |
| Platelets (PLT) | [pg] | 763.5 ± 27.4 | 801.6 ± 52.8 | 178.14 ± 62.5 | 218.7 ± 23.0 |
| LYM% | [%] | 72.22 ± 1.52 | 37.83 ± 2.80 | 55.96 ± 3.82 | 60.9 ± 3.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Na, N.T.; Duc Loc, S.; Minh Tri, N.L.; Bich Loan, N.T.; Anh Son, H.; Linh Toan, N.; Phuong Thu, H.; My Nhung, H.T.; Lai Thanh, N.; Van Anh, N.T.; et al. Nanomelanin Potentially Protects the Spleen from Radiotherapy-Associated Damage and Enhances Immunoactivity in Tumor-Bearing Mice. Materials 2019, 12, 1725. https://doi.org/10.3390/ma12101725
Le Na NT, Duc Loc S, Minh Tri NL, Bich Loan NT, Anh Son H, Linh Toan N, Phuong Thu H, My Nhung HT, Lai Thanh N, Van Anh NT, et al. Nanomelanin Potentially Protects the Spleen from Radiotherapy-Associated Damage and Enhances Immunoactivity in Tumor-Bearing Mice. Materials. 2019; 12(10):1725. https://doi.org/10.3390/ma12101725
Chicago/Turabian StyleLe Na, Nguyen Thi, Sai Duc Loc, Nguyen Le Minh Tri, Nguyen Thi Bich Loan, Ho Anh Son, Nguyen Linh Toan, Ha Phuong Thu, Hoang Thi My Nhung, Nguyen Lai Thanh, Nguyen Thi Van Anh, and et al. 2019. "Nanomelanin Potentially Protects the Spleen from Radiotherapy-Associated Damage and Enhances Immunoactivity in Tumor-Bearing Mice" Materials 12, no. 10: 1725. https://doi.org/10.3390/ma12101725
APA StyleLe Na, N. T., Duc Loc, S., Minh Tri, N. L., Bich Loan, N. T., Anh Son, H., Linh Toan, N., Phuong Thu, H., My Nhung, H. T., Lai Thanh, N., Van Anh, N. T., & Dinh Thang, N. (2019). Nanomelanin Potentially Protects the Spleen from Radiotherapy-Associated Damage and Enhances Immunoactivity in Tumor-Bearing Mice. Materials, 12(10), 1725. https://doi.org/10.3390/ma12101725