Faster Release of Lumen-Loaded Drugs than Matrix-Loaded Equivalent in Polylactic Acid/Halloysite Nanotubes
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Methods
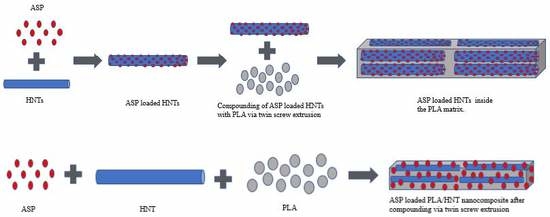
2.2.1. Drug Loading of HNTs
2.2.2. Preparation of PLA/HNT/ASP Nanocomposites
2.2.3. Characterisation of the Drug-Loaded Nanocomposite
Differential Scanning Calorimetry (DSC)
Mechanical Testing
Fourier Transform Infrared (FTIR) Spectroscopy
Goniometry (Surface Wettability)
Evaluation of Drug-Loaded Efficiency
In Vitro Drug Release Properties
Statistical Analysis
3. Results
3.1. Preparation of Drug-Loaded HNTs
3.2. Processing of PLA and HNT through Melt Extrusion
3.3. Differential Scanning Calorimetry (DSC)
3.4. Mechanical Testing
3.5. Fourier Transfer Infrared Spectroscopy (FTIR)
3.6. Surface Wettability
3.7. Drug Release
4. Discussion
4.1. Drug Loading
4.2. Melt Extrusion
4.3. Thermal Characteristics
4.4. Mechanical Properties
4.5. Fourier Transform Infrared (FTIR) Spectroscopy
4.6. Surface Wettability
4.7. Drug Release
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| HME | Hot-melt extrusion |
| API | Active pharmaceutical ingredients |
| PLA | Polylactic acid |
| HNTs | Halloysite nanotubes |
| ASP | Aspirin (acetyl salicylic acid) |
| Lumen loading | ASP loading on HNTs and processing with PLA |
| Matrix loading | ASP, HNTs and PLA are mixed and processed |
| B1 | 1:1 ratio of lumen loading |
| B2 | 1:1 ratio of matrix loading |
| B3 | 2:1 ratio of matrix loading |
| B4 | Processed PLA/HNT nanocomposite (matrix loading) |
| B5 | 2:1 ratio of lumen loading |
| B6 | Processed virgin PLA (matrix loading) |
| DSC | Differential scanning calorimetry |
| Tg | Glass transition temperature |
| Tcc | Cold crystallisation temperature |
| Tm | Melting temperature |
| FTIR | Fourier transfer infrared spectroscopy |
| SEM | Scanning electron microscopy |
References
- Fule, R.; Paithankar, V.; Amin, P. Hot melt extrusion based solid solution approach: Exploring polymer comparison, physicochemical characterization and in-vivo evaluation. Inter. J. Pharm. 2016, 499, 280–294. [Google Scholar] [CrossRef]
- Lyons, J.G.; Holehonnur, H.; Devine, D.M.; Kennedy, J.E.; Geever, L.M.; Blackie, P.; Higginbotham, C.L. The incorporation of an organically modified layered silicate in monolithic polymeric matrices produced using hot melt extrusion. Mater. Chem. Phys. 2007, 103, 419–426. [Google Scholar] [CrossRef]
- Martinez-Marcos, L.; Lamprou, D.A.; McBurney, R.T.; Halbert, G.W. A novel hot-melt extrusion formulation of albendazole for increasing dissolution properties. Inter. J. Pharm. 2016, 499, 175–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-Melt Extrusion: From Theory to Application in Pharmaceutical Formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process technology to pharmaceutical products. ISRN Pharm. 2012, 2012, 436763–436769. [Google Scholar] [CrossRef] [PubMed]
- Saerens, L.; Vervaet, C.; Remon, J.P.; De Beer, T. Process monitoring and visualization solutions for hot-melt extrusion: A review. J. Pharm. Pharmacol. 2014, 66, 180–203. [Google Scholar] [CrossRef]
- Healy, A.V.; Waldron, C.; Geever, L.M.; Devine, D.M.; Lyons, J.G. Degradable Nanocomposites for Fused Filament Fabrication Applications. J. Manuf. Mater. Process. 2018, 2, 29. [Google Scholar] [CrossRef]
- Chen, Y.; Murphy, A.; Scholz, D.; Geever, L.M.; Lyons, J.G.; Devine, D.M. Surface-modified halloysite nanotubes reinforced poly(lactic acid) for use in biodegradable coronary stents. J. Appl. Polym. Sci. 2018a, 135, 46521. [Google Scholar] [CrossRef]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Review of Multifarious Applications of Poly (Lactic Acid). Polym. Plastics Technol. Eng. 2016, 55. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications — A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Mhlanga, N.; Ray, S.S. Kinetic models for the release of the anticancer drug doxorubicin from biodegradable polylactide/metal oxide-based hybrids. Inter. J. Biol. Macromol. 2015, 72, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different Techniques for preparation of polymeric nanoparticles—A review. Asian J. Pharm. Clinical Res. 2012, 5, 16–23. [Google Scholar]
- Sha, L.; Chen, Z.; Chen, Z.; Zhang, A.; Yang, Z. Polylactic acid based nanocomposites: Promising safe and biodegradable materials in biomedical field. Inter. J. Polym. Sci. 2016. [Google Scholar] [CrossRef]
- Laboratory of Polymeric and Composite Materials. Available online: http://morris.umh.ac.be/smpc/poster.aspx (accessed on 27 May 2019).
- Prashantha, K.; Lacrampe, M.-F.; Krawczak, P. Halloysite Nanotubes-Polymer Nano composites:A New Class of Multifaceted Materials. Advan. Mater. Manuf. Charact. 2013, 3, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Cao, X.; Zhang, Y.; He, G. Polylactide/halloysite nanotube nanocomposites: Thermal, mechanical properties, and foam processing. J. Appl. Polym. Sci. 2013, 130, 443–452. [Google Scholar] [CrossRef]
- Castro-aguirre, E.; Auras, R.; Selke, S.; Rubino, M.; Marsh, T. Impact of Nanoclays on the Biodegradation of Poly (lactic acid) Nanocomposites. Polymers 2018, 10, 202. [Google Scholar] [CrossRef]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Halloysite nanotube reinforced polylactic acid composite. Polym. Compos. 2017, 38. [Google Scholar] [CrossRef]
- Gaaz, T.; Sulong, A.; Kadhum, A.; Al-Amiery, A.; Nassir, M.; Jaaz, A. The Impact of Halloysite on the Thermo-Mechanical Properties of Polymer Composites. Molecules 2017, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The application of halloysite tubule nanoclay in drug delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Lvov, Y. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. J. Nanosci. Nanotechnol. 2011, 11, 10007–10026. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yang, H.; Tang, A.; Hu, Y. Engineering a tubular mesoporous silica nanocontainer with well-preserved clay shell from natural halloysite. Nano Res. 2017, 10, 2782–2799. [Google Scholar] [CrossRef]
- Leporatti, S. Halloysite clay nanotubes as nano-bazookas for drug delivery. Polym. Inter. 2017, 66, 1111–1118. [Google Scholar] [CrossRef]
- Lun, H.; Ouyang, J.; Yang, H. Natural halloysite nanotubes modified as an aspirin carrier. RSC Adv. 2014, 4, 44197–44202. [Google Scholar] [CrossRef]
- Patel, S.; Jammalamadaka, U.; Sun, L.; Tappa, K.; Mills, D. Sustained Release of Antibacterial Agents from Doped Halloysite Nanotubes. Bioengineering 2015, 3, 1. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Shen, M.; Cao, X.; Zhang, L.; Xu, J.; Yu, J.; Shi, X. Electrospun poly(lactic-co-glycolic acid)/halloysite nanotube composite nanofibers for drug encapsulation and sustained release. J. Mater. Chem. 2010, 20, 10622. [Google Scholar] [CrossRef]
- Katerina, M. Aspirin–friend or foe? THTY 2008, 1, 150–151. [Google Scholar]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cao, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef] [Green Version]
- Ng, J.C.; Yeomans, N.D. Infection and the risk of upper gastrointestinal bleeding in low dose aspirin users: Systematic review and meta-analysis. Med. J. Aust. 2018, 209, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Singh, J. Controlled delivery of aspirin: Effect of aspirin on polymer degradation and in vitro release from PLGA based phase sensitive systems. Inter. J. Pharm. 2008, 357, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Gene, H. Guided bone regeneration with asymmetric collagen-chitosan membranes containing aspirin- loaded chitosan nanoparticles. Inter. J. Nanomed. 2017, 12, 8855–8866. [Google Scholar] [CrossRef] [PubMed]
- Devine, D.M.; Geever, L.M.; Higginbotham, C.L. Drug release from a N-vinylpyrrolidinone/acrylic acid lubricious hydrophilic coatin. J. Mater. Sci. 2005, 40, 3429–3436. [Google Scholar] [CrossRef]
- Devine, D.M.; Devery, S.M.; Lyons, J.G.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L. Multifunctional polyvinylpyrrolidinone-polyacrylic acid copolymer hydrogels for biomedical applications. Inter. J. Pharm. 2006, 326, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wan, A.; Shi, Y.; Zhang, Y.; Chen, Y. Experimental and mathematical studies on the drug release properties of aspirin loaded chitosan nanoparticles. BioMed. Res. Inter. 2014, 2014. [Google Scholar] [CrossRef]
- Das, S.; Banerjee, R.; Bellare, J. Aspirin loaded albumin nanoparticles by coacervation: Implications in drug delivery. Trends Biomater. Artif. Org. 2005, 18, 203–212. [Google Scholar]
- Garlotta, D. A Literature Review of Poly (Lactic Acid). J. Polym. Environ. 2002, 9, 63–84. [Google Scholar] [CrossRef]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M.S.M. Development and Characterization of Aspirin-Phospholipid Complex for Improved Drug Delivery. J. Pharm. Sci. 2010, 3, 940–947. [Google Scholar]
- Višnjić, D.; Lalić, H.; Dembitz, V.; Banfić, H. Metabolism and differentiation. Period. Biol. 2014, 116, 37–43. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Zheng, F.; Liu, H.; Yu, J.; Shi, X. Controlled release and antibacterial activity of antibiotic-loaded electrospun halloysite/poly(lactic-co-glycolic acid) composite nanofibers. Coll. Surf. B Biointerfaces 2013, 110, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, C.; Chen, Y.; Cao, Z.; Brennan, S.; Major, I.; Lyons, J.; Devine, D. Increased screw speed has positive effect on Polylactic Acid-Halloysite Nanotubes nanocomposite. In submission.
- Jani, R.; Patel, D. Hot melt extrusion: An industrially feasible approach for casting orodispersible film. Asian J. Pharm. Sci. 2014, 10, 292–305. [Google Scholar] [CrossRef]
- Gao, M.; Lu, L.; Wang, X.; Lin, H.; Zhou, Q. Preparation of a novel breviscapine-loaded halloysite nanotubes complex for controlled release of breviscapine Preparation of a novel breviscapine-loaded halloysite nanotubes complex for controlled release of breviscapine. Mater. Sci. Eng. 2017, 265, 1–8. [Google Scholar] [CrossRef]
- Balogh, A.; Domokos, A.; Farkas, B.; Farkas, A.; Rapi, Z.; Kiss, D. Supporting Information Continuous End-to-End Production of Solid Drug Dosage Forms: Coupling Flow Synthesis and Formulation by Electrospinning. Chem. Eng. J. 2018, 350, 1–23. [Google Scholar] [CrossRef]
- Devine, D.M.; Hoctor, E.; Hayes, J.S.; Sheehan, E.; Evans, C.H. Extended release of proteins following encapsulation in hydroxyapatite/chitosan composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2017, 84, 281–289. [Google Scholar] [CrossRef]
- Dong, Y.; Marshall, J.; Haroosh, H.J.; Mohammadzadehmoghadam, S.; Liu, D.; Qi, X.; Lau, K.T. Polylactic acid (PLA)/halloysite nanotube (HNT) composite mats: Influence of HNT content and modification. Compos. Part A Appl. Sci. Manuf. 2015, 76, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Zhang, G.; Phuong, T.V.; Lazzeri, A. Synergistic effects of nucleating agents and plasticizers on the crystallization behavior of Poly(lactic acid). Molecules 2015, 20, 1579–1593. [Google Scholar] [CrossRef]
- Lazzara, G.; Massaro, M.; Milioto, S.; Riela, S. Halloysite-based bionanocomposites. Handb. Compos. Renew. Mater. 2017, 1–8, 557–584. [Google Scholar] [CrossRef]












| Batch | Type of Loading | Composition | HNTs:ASP Ratio |
|---|---|---|---|
| B1 | Lumen-loaded | 95% PLA, 5% (HNT+ASP) | 1:1 |
| B5 | Lumen-loaded | 95% PLA, 5% (HNT+ASP) | 2:1 |
| B2 | Matrix-loaded | 95% PLA, 5% (HNT+ASP) | 1:1 |
| B3 | Matrix-loaded | 95% PLA, 5% (HNT+ASP) | 2:1 |
| B4 | Matrix-loaded | 95% PLA, 5% HNT | - |
| B6 | Matrix-loaded | 100%PLA | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, C.; Clear, O.; Major, I.; Lyons, J.G.; Devine, D.M. Faster Release of Lumen-Loaded Drugs than Matrix-Loaded Equivalent in Polylactic Acid/Halloysite Nanotubes. Materials 2019, 12, 1830. https://doi.org/10.3390/ma12111830
Venkatesh C, Clear O, Major I, Lyons JG, Devine DM. Faster Release of Lumen-Loaded Drugs than Matrix-Loaded Equivalent in Polylactic Acid/Halloysite Nanotubes. Materials. 2019; 12(11):1830. https://doi.org/10.3390/ma12111830
Chicago/Turabian StyleVenkatesh, Chaitra, Oran Clear, Ian Major, John G. Lyons, and Declan M. Devine. 2019. "Faster Release of Lumen-Loaded Drugs than Matrix-Loaded Equivalent in Polylactic Acid/Halloysite Nanotubes" Materials 12, no. 11: 1830. https://doi.org/10.3390/ma12111830
APA StyleVenkatesh, C., Clear, O., Major, I., Lyons, J. G., & Devine, D. M. (2019). Faster Release of Lumen-Loaded Drugs than Matrix-Loaded Equivalent in Polylactic Acid/Halloysite Nanotubes. Materials, 12(11), 1830. https://doi.org/10.3390/ma12111830