One Step Preparation of Peptide-Coated Gold Nanoparticles with Tunable Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Protocol for One-Step Preparation
- Method A: 2 mL of tyrosine was mixed with 0.9 mL CALNN, and then 0.1 mL chloroauric acid was added, quickly mixed and reacted at 37 °C for 2 h with gentle rotation.
- Method B: 2 mL of tyrosine was mixed with 0.1 mL chloroauric acid, and then 0.9 mL CALNN was added, and the mixture reacted at 37 °C for 2 h with gentle rotation.
- Method C: 2 mL of tyrosine was added to the mixture of 0.9 mL CALNN and 0.1 mL chloroauric acid, mixed well and reacted at 37 °C for 2 h with gentle rotation.
2.3. Preparation of CALNN-Coated AuNPs with Different Size
2.4. Preparation of Functional AuNPs
2.5. Determination of Trypsin
2.6. Characterization
2.6.1. Transmission Electron Microscopy (TEM)
2.6.2. UV–Visible Absorption Spectroscopy
2.6.3. Thermogravimetric Analysis (TGA)
2.6.4. Dynamic Light Scattering (DLS)
2.6.5. Fluorescence Spectroscopy
2.6.6. Energy dispersive X-ray spectroscopy (EDS)
3. Results and Discussion
3.1. AuNP Preparation
3.2. Optimization
3.3. Characterization
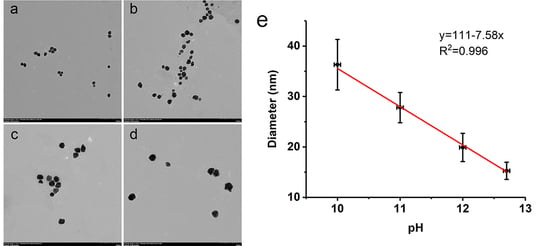
3.4. Size Control
3.5. Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lévy, R. Peptide-Capped Gold Nanoparticles: Towards Artificial Proteins. ChemBioChem 2006, 7, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Vignoni, M.; de Alwis Weerasekera, H.; Simpson, M.J.; Phopase, J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I.; Scaiano, J.C. LL37 peptide@silver nanoparticles: Combining the best of the two worlds for skin infection control. Nanoscale 2014, 6, 5725–5728. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, M.; Jacques, E.; Andronic, C.; Comer, J.; Poblete, H.; Alarcon, E.I. Novel specific peptides as superior surface stabilizers for silver nano structures: Role of peptide chain length. J. Mater. Chem. B 2017, 5, 8925–8928. [Google Scholar] [CrossRef]
- Galbiati, E.; Gambini, L.; Civitarese, V.; Bellini, M.; Ambrosini, D.; Allevi, R.; Avvakumova, S.; Romeo, S.; Prosperi, D. “Blind” targeting in action: From phage display to breast cancer cell targeting with peptide-gold nanoconjugates. Pharmacol. Res. 2016, 111, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Chu, X.; Chen, T.; Ge, J.; Yu, R. Graphene Oxide–Peptide Conjugate as an Intracellular Protease Sensor for Caspase-3 Activation Imaging in Live Cells. Angew. Chem. Int. Ed. 2011, 50, 7065–7069. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, H.; Shen, S.; She, X.; Shi, W.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. Fibrin-targeting peptide CREKA-conjugated multi-walled carbon nanotubes for self-amplified photothermal therapy of tumor. Biomaterials 2016, 79, 46–55. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Xu, X.; Xia, X.; Wang, H.; Li, L.; Dong, W.; Ma, P.; Yang, Y.; Liu, Y.; et al. Thermal and magnetic dual-responsive liposomes with a cell-penetrating peptide-siRNA conjugate for enhanced and targeted cancer therapy. Colloids Surf. B 2016, 146, 607–615. [Google Scholar] [CrossRef]
- Das, P.; Fatehbasharzad, P.; Colombo, M.; Fiandra, L.; Prosperi, D. Multifunctional Magnetic Gold Nanomaterials for Cancer. Trends Biotechnol. 2019. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.-Q.; Ye, B.-C. Colorimetric assay for parallel detection of Cd2+, Ni2+ and Co2+ using peptide-modified gold nanoparticles. Analyst 2012, 137, 601–607. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Z.; Hu, L.; Yuan, Y.; Xu, G. Turn-On Fluorescence Sensor Based on Single-Walled-Carbon-Nanohorn–Peptide Complex for the Detection of Thrombin. Chem. Eur. J. 2012, 18, 16556–16561. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Li, Q.; Wu, Z.; Jiang, J.-H.; Yu, R.-Q. Graphene oxide–peptide nanoassembly as a general approach for monitoring the activity of histone deacetylases. Analyst 2016, 141, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Wang, N.; Li, J.; Yang, R. Peptide-fluorophore/AuNP conjugate-based two-photon excited fluorescent nanosensor for caspase-3 activity imaging assay in living cells and tissue. MedChemComm 2017, 8, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Stewart, M.H.; Deschamps, J.R.; Susumu, K.; Oh, E.; Tsytsarev, V.; Tang, Q.; Efros, A.L.; Vaxenburg, R.; Black, B.J.; et al. Quantum Dot–Peptide–Fullerene Bioconjugates for Visualization of in Vitro and in Vivo Cellular Membrane Potential. ACS Nano 2017, 11, 5598–5613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, Y.; Luo, M.; Li, X.; Xu, Q.; Ji, X.; He, Z. Controlled Assembly of Gold Nanoparticles through Antibody Recognition: Study and Utilizing the Effect of Particle Size on Interparticle Distance. Langmuir 2013, 29, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Coomber, D.; Bartczak, D.; Gerrard, S.R.; Tyas, S.; Kanaras, A.G.; Stulz, E. Programmed Assembly of Peptide-Functionalized Gold Nanoparticles on DNA Templates. Langmuir 2010, 26, 13760–13762. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Sedighi, A.; Krull, U.J. Cancer biomarker determination by resonance energy transfer using functional fluorescent nanoprobes. Anal. Chim. Acta 2018, 1041, 1–24. [Google Scholar] [CrossRef]
- Kramer, R.M.; Li, C.; Carter, D.C.; Stone, M.O.; Naik, R.R. Engineered Protein Cages for Nanomaterial Synthesis. J. Am. Chem. Soc. 2004, 126, 13282–13286. [Google Scholar] [CrossRef]
- Lévy, R.; Thanh, N.T.K.; Doty, R.C.; Hussain, I.; Nichols, R.J.; Schiffrin, D.J.; Brust, M.; Fernig, D.G. Rational and Combinatorial Design of Peptide Capping Ligands for Gold Nanoparticles. J. Am. Chem. Soc. 2004, 126, 10076–10084. [Google Scholar] [CrossRef]
- Shaw, C.P.; Middleton, D.A.; Volk, M.; Lévy, R. Amyloid-Derived Peptide Forms Self-Assembled Monolayers on Gold Nanoparticle with a Curvature-Dependent β-Sheet Structure. ACS Nano 2012. [Google Scholar] [CrossRef]
- Li, X.-Y.; Feng, F.-Y.; Zhou, X.-D.; Hu, J.-M. Rational design of an anchoring peptide for high-efficiency and quantitative modification of peptides and DNA strands on gold nanoparticles. Nanoscale 2018, 10, 11491–11497. [Google Scholar] [CrossRef] [PubMed]
- Dalton, A.B.; Ortiz-Acevedo, A.; Zorbas, V.; Brunner, E.; Sampson, W.M.; Collins, S.; Razal, J.M.; Miki Yoshida, M.; Baughman, R.H.; Draper, R.K.; et al. Hierarchical Self-Assembly of Peptide-Coated Carbon Nanotubes. Adv. Funct. Mater. 2004, 14, 1147–1151. [Google Scholar] [CrossRef]
- Wang, S.; Humphreys, E.S.; Chung, S.-Y.; Delduco, D.F.; Lustig, S.R.; Wang, H.; Parker, K.N.; Rizzo, N.W.; Subramoney, S.; Chiang, Y.-M.; et al. Peptides with selective affinity for carbon nanotubes. Nat. Mater. 2003, 2, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, G.R.; Dalton, A.B.; Johnson, P.A.; Razal, J.; Chen, J.; Giordano, G.M.; Muñoz, E.; Musselman, I.H.; Baughman, R.H.; Draper, R.K. Controlled Assembly of Carbon Nanotubes by Designed Amphiphilic Peptide Helices. J. Am. Chem. Soc. 2003, 125, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.R.; Jones, S.E.; Murray, C.J.; McAuliffe, J.C.; Vaia, R.A.; Stone, M.O. Peptide Templates for Nanoparticle Synthesis Derived from Polymerase Chain Reaction-Driven Phage Display. Adv. Funct. Mater. 2004, 14, 25–30. [Google Scholar] [CrossRef]
- Pinaud, F.; King, D.; Moore, H.-P.; Weiss, S. Bioactivation and Cell Targeting of Semiconductor CdSe/ZnS Nanocrystals with Phytochelatin-Related Peptides. J. Am. Chem. Soc. 2004, 126, 6115–6123. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhu, E.; Liu, J.; Zhang, S.; Lin, Z.; Duan, X.; Heinz, H.; Huang, Y.; De Yoreo, J.J. Building two-dimensional materials one row at a time: Avoiding the nucleation barrier. Science 2018, 362, 1135–1139. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.-R.; Chen, Y.-L.; Wang, L.; Wang, W.-F.; Chen, X.-G. Highly sensitive fluorescence detection of trypsin based on gold nanoparticle probes. Anal. Methods 2016, 8, 393–400. [Google Scholar] [CrossRef]
- Chandrawati, R.; Stevens, M.M. Controlled assembly of peptide-functionalized gold nanoparticles for label-free detection of blood coagulation Factor XIII activity. Chem. Commun. 2014, 50, 5431–5434. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Y.; Chen, P.; McCadden, A.; Palaniappan, A.; Zhang, J.; Liedberg, B. Peptide Functionalized Gold Nanoparticles with Optimized Particle Size and Concentration for Colorimetric Assay Development: Detection of Cardiac Troponin I. ACS Sens. 2016, 1, 1416–1422. [Google Scholar] [CrossRef]
- Liu, L.; Xia, N.; Liu, H.; Kang, X.; Liu, X.; Xue, C.; He, X. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, D.; Wang, Z. Functional Gold Nanoparticle−Peptide Complexes as Cell-Targeting Agents. Langmuir 2008, 24, 10293–10297. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Soares, M.E.; Duarte, J.A.; Soares, L.; Maia, S.; Gomes, P.; Pereira, E.; Fraga, S.; Carmo, H.; Bastos, M.D.L. Effect of surface coating on the biodistribution profile of gold nanoparticles in the rat. Eur. J. Pharm. Biopharm. 2012, 80, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Chen, C.; Zhang, L.; Guo, X.; Wang, H.; Ji, X.; He, Z. Robust Aqueous Quantum Dots Capped with Peptide Ligands as Biomaterials: Facile Preparation, Good Stability, and Multipurpose Application. Part. Part. Syst. Charact. 2014, 31, 382–389. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, H.; Zhou, Y.; Liu, P.; Jia, Y.; Ye, C. Peptide-coated palladium nanoparticle for highly sensitive bioanalysis of trypsin in human urine samples. Nanomater. Nanotechnol. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Sirajuddin; Mechler, A.; Torriero, A.A.J.; Nafady, A.; Lee, C.-Y.; Bond, A.M.; O’Mullane, A.P.; Bhargava, S.K. The formation of gold nanoparticles using hydroquinone as a reducing agent through a localized pH change upon addition of NaOH to a solution of HAuCl4. Colloids Surf. A 2010, 370, 35–41. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Khlebtsov, N.G. Determination of Size and Concentration of Gold Nanoparticles from Extinction Spectra. Anal. Chem. 2008, 80, 6620–6625. [Google Scholar] [CrossRef]
- Kamble, G.S.; Kolekar, S.S.; Han, S.H.; Anuse, M.A. Synergistic liquid–liquid extractive spectrophotometric determination of gold(III) using 1-(2′,4′-dinitro aminophenyl)-4,4,6-trimethyl-1,4-dihydropyrimidine-2-thiol. Talanta 2010, 81, 1088–1095. [Google Scholar] [CrossRef]
- Celentano, M.; Jakhmola, A.; Profeta, M.; Battista, E.; Guarnieri, D.; Gentile, F.; Netti, P.A.; Vecchione, R. Diffusion limited green synthesis of ultra-small gold nanoparticles at room temperature. Colloids Surf. A 2018, 558, 548–557. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Yan, X.; Guo, X.; Zhou, G.; Liu, P.; Li, Z. One Step Preparation of Peptide-Coated Gold Nanoparticles with Tunable Size. Materials 2019, 12, 2107. https://doi.org/10.3390/ma12132107
Jia Y, Yan X, Guo X, Zhou G, Liu P, Li Z. One Step Preparation of Peptide-Coated Gold Nanoparticles with Tunable Size. Materials. 2019; 12(13):2107. https://doi.org/10.3390/ma12132107
Chicago/Turabian StyleJia, Yongmei, Xiaoning Yan, Xin Guo, Guohua Zhou, Peilian Liu, and Zhiguo Li. 2019. "One Step Preparation of Peptide-Coated Gold Nanoparticles with Tunable Size" Materials 12, no. 13: 2107. https://doi.org/10.3390/ma12132107
APA StyleJia, Y., Yan, X., Guo, X., Zhou, G., Liu, P., & Li, Z. (2019). One Step Preparation of Peptide-Coated Gold Nanoparticles with Tunable Size. Materials, 12(13), 2107. https://doi.org/10.3390/ma12132107