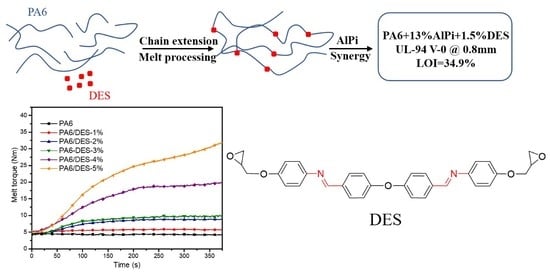
Chain Extension and Synergistic Flame-Retardant Effect of Aromatic Schiff Base Diepoxide on Polyamide 6/Aluminum Diethylphosphinate Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of DES
2.3. Instruments and Methods
2.4. Processing and Specimen Preparation
3. Results and Discussion
3.1. Chain Extension Effect
3.2. Mechanical Properties and Crystallinity
3.3. Flame Retardancy
3.4. Cone Calorimeter Test
3.5. Residue Analysis
3.6. Thermal Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weil, E.D.; Levchik, S. Current practice and recent commercial developments in flame retardancy of polyamides. J. Fire Sci. 2004, 22, 251–264. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Fei, G.X.; Liu, Y.; Wang, Q. Synergistic effects of novolac-based char former with magnesium hydroxide in flame retardant polyamide-6. Polym. Degrad. Stab. 2008, 93, 1351–1356. [Google Scholar] [CrossRef]
- Arjmandi, R.; Balakrishnan, H.; Hassan, A.; Jawaid, M.; Othman, A.Y. Enhanced flame retardancy, thermal and mechanical properties of hybrid magnesium hydroxide/montmorillonite reinforced polyamide 6/polypropylene nanocomposites. Fiber. Polym. 2018, 19, 914–926. [Google Scholar] [CrossRef]
- Balakrishnan, H.; Hassan, A.; Isitman, N.A.; Kaynak, C. On the use of magnesium hydroxide towards halogen-free flame-retarded polyamide-6/polypropylene blends. Polym. Degrad. Stab. 2012, 97, 1447–1457. [Google Scholar] [CrossRef]
- Balabanovich, A.I.; Schnabel, W. Fire retardance in polyamide-6,6. The effects of red phosphorus and radiation-induced cross-links. Macromol. Mater. Eng. 2002, 287, 187–194. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q. Preparation of microencapsulated red phosphorus through melamine cyanurate self-assembly and its performance in flame retardant polyamide 6. Polym. Eng. Sci. 2006, 46, 1548–1553. [Google Scholar]
- Zhao, M.; Yi, D.Q.; Yang, R.J. Enhanced mechanical properties and fire retardancy of polyamide 6 nanocomposites based on interdigitated crystalline montmorillonite-melamine cyanurate. J. Appl. Polym. Sci. 2018, 135, 46039–46047. [Google Scholar] [CrossRef]
- Tao, W.; Li, J. Melamine cyanurate tailored by base and its multi effects on flame retardancy of polyamide 6. Appl. Surf. Sci. 2018, 456, 751–762. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, K.; Xiao, R. Preparation and characterization of flame-retarded polyamide 66 with melamine cyanurate by in situ polymerization. Macromol. Res. 2017, 25, 779–785. [Google Scholar] [CrossRef]
- Tai, Q.; Yuen, R.K.K.; Yang, W.; Qiao, Z.; Song, L.; Hu, Y. Iron-montmorillonite and zinc borate as synergistic agents in flame-retardant glass fiber reinforced polyamide 6 composites in combination with melamine polyphosphate. Compos. Pt. A-Appl. Sci. Manuf. 2012, 43, 415–422. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wang, Q. The investigation of melamine polyphosphate flame retardant polyamide-6/inorganic siliciferous filler with different geometrical form. J. Appl. Polym. Sci. 2009, 113, 2046–2051. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Wang, Q. Melamine polyphosphate/silicon-modified phenolic resin flame retardant glass fiber reinforced polyamide-6. J. Appl. Polym. Sci. 2013, 129, 2171–2176. [Google Scholar] [CrossRef]
- Li, L.L.; Chen, S.H.; Ma, W.J.; Cheng, Y.H.; Tao, Y.P.; Wu, T.Z.; Chen, W.P.; Zhou, Z.; Zhu, M.F. A novel reduced graphene oxide decorated with halloysite nanotubes (hnts-d-rgo) hybrid composite and its flame-retardant application for polyamide 6. Express Polym. Lett. 2014, 8, 450–457. [Google Scholar] [CrossRef]
- Li, L.L.; Wu, Z.H.; Jiang, S.S.; Zhang, S.D.; Lu, S.Y.; Chen, W.P.; Sun, B.; Zhu, M.F. Effect of halloysite nanotubes on thermal and flame retardant properties of polyamide 6/melamine cyanurate composites. Polym. Compos. 2015, 36, 892–896. [Google Scholar] [CrossRef]
- Hao, A.; Wong, I.; Wu, H.; Lisco, B.; Ong, B.; Sallean, A.; Butler, S.; Londa, M.; Koo, J.H. Mechanical, thermal, and flame-retardant performance of polyamide 11-halloysite nanotube nanocomposites. J. Mater. Sci. 2015, 50, 157–167. [Google Scholar] [CrossRef]
- Lei, S.; Yuan, H.; Lin, Z.H.; Xuan, S.Y.; Wang, S.F.; Chen, Z.Y.; Fan, W.C. Preparation and properties of halogen-free flame-retarded polyamide 6/organoclay nanocomposite. Polym. Degrad. Stab. 2004, 86, 535–540. [Google Scholar]
- Wu, X.F.; Zhao, Y.K.; Zhao, Z.H.; Sun, Y.; Zhang, H.; Yu, M.T.; Jia, F.F. Crystallization behaviors of graphene oxide-carbon nanotubes hybrids/polyamide 66 composites. Polym.-Plast. Technol. Eng. 2017, 56, 556–562. [Google Scholar] [CrossRef]
- Sehic, A.; Vasiljevic, J.; Demsar, A.; Leskovsek, M.; Bukosek, V.; Medved, J.; Colovic, M.; Jerman, I.; Simoncic, B. Polyamide 6 composite fibers with incorporated mixtures of melamine cyanurate, carbon nanotubes, and carbon black. J. Appl. Polym. Sci. 2019, 136, 47007–47017. [Google Scholar] [CrossRef]
- Cao, Y.F.; Qian, L.J.; Chen, Y.J.; Wang, Z. Synergistic flame-retardant effect of phosphaphenanthrene derivative and aluminum diethylphosphinate in glass fiber reinforced polyamide 66. J. Appl. Polym. Sci. 2017, 134, 45126–45133. [Google Scholar] [CrossRef]
- Ma, K.; Li, B.; Xu, M.J. Simultaneously improving the flame retardancy and mechanical properties for polyamide 6/aluminum diethylphosphinate composites by incorporating of 1,3,5-triglycidyl isocyanurate. Polym. Adv. Technol. 2018, 29, 1068–1077. [Google Scholar] [CrossRef]
- Feng, H.S.; Qiu, Y.; Qian, L.J.; Chen, Y.J.; Xu, B.; Xin, F. Flame inhibition and charring effect of aromatic polyimide and aluminum diethylphosphinate in polyamide 6. Polymers 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Polat, O.; Kaynak, C. Use of boron oxide and boric acid to improve flame retardancy of an organophosphorus compound in neat and fiber reinforced polyamide-6. J. Vinyl Addit. Technol. 2016, 22, 300–310. [Google Scholar] [CrossRef]
- Kaynak, C.; Polat, O. Influences of nanoclays on the flame retardancy of fiber-filled and unfilled polyamide-6 with and without aluminum diethylphosphinate. J. Fire Sci. 2015, 33, 87–112. [Google Scholar] [CrossRef]
- Wirasaputra, A.; Zheng, L.; Liu, S.; Yuan, Y.; Zhao, J. High-performance flame-retarded polyamide-6 composite fabricated by chain extension. Macromol. Mater. Eng. 2016, 301, 614–624. [Google Scholar] [CrossRef]
- Xu, M.J.; Ma, K.; Jiang, D.W.; Zhang, J.X.; Zhao, M.; Guo, X.K.; Shao, Q.; Wujcik, E.; Li, B.; Guo, Z.H. Hexa-[4-(glycidyloxycarbonyl) phenoxy]cyclotriphosphazene chain extender for preparing high-performance flame retardant polyamide 6 composites. Polymer 2018, 146, 63–72. [Google Scholar] [CrossRef]
- Xu, M.L.; Yan, H.C.; He, Q.J.; Wan, C.; Liu, T.; Zhao, L.; Park, C.B. Chain extension of polyamide 6 using multifunctional chain extenders and reactive extrusion for melt foaming. Eur. Polym. J. 2017, 96, 210–220. [Google Scholar] [CrossRef]
- Lu, C.X.; Ye, R.G.; Yang, Y.; Ren, X.C.; Cai, X.F. Chemical modification of polyamide 6 by chain extension with terephthaloyl-biscaprolactam. J. Macromol. Sci. Part B-Phys. 2011, 50, 350–362. [Google Scholar] [CrossRef]
- Lu, C.; Chen, L.; Ye, R.; Cai, X. Chain extension of polyamide 6 using bisoxazoline coupling agents. J. Macromol. Sci. Part B-Phys. 2008, 47, 986–999. [Google Scholar] [CrossRef]
- Néry, L.; Lefebvre, H.; Fradet, A. Chain extension of carboxy-terminated aliphatic polyamides and polyesters by arylene and pyridylene bisoxazolines. Macromol. Chem. Phys. 2004, 205, 448–455. [Google Scholar] [CrossRef]
- Buccella, M.; Dorigato, A.; Caldara, M.; Pasqualini, E.; Fambri, L. Thermo-mechanical behaviour of polyamide 6 chain extended with 1,1′-carbonyl-bis-caprolactam and 1,3-phenylene-bis-2-oxazoline. J. Polym. Res. 2013, 20, 225–233. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, X.; Xu, J.; Guo, B. Chain extension of pa1010 by reactive extrusion by diepoxide 711 and diepoxide tde85 as chain extenders. J. Appl. Polym. Sci. 2004, 94, 2347–2355. [Google Scholar] [CrossRef]
- Cai, J.N.; Wirasaputra, A.; Zhu, Y.M.; Liu, S.; Zhou, Y.; Zhang, C.; Zhao, J. The flame retardancy and rheological properties of pa6/mca modified by dopo-based chain extender. RSC Adv. 2017, 7, 19593–19603. [Google Scholar] [CrossRef]
- Han, X.; Zhao, J.; Liu, S.; Yuan, Y. Flame retardancy mechanism of poly(butylene terephthalate)/aluminum diethylphosphinate composites with an epoxy-functional polysiloxane. RSC Adv. 2014, 4, 16551–16560. [Google Scholar] [CrossRef]
- Zhang, P.K.; Fan, H.J.; Tian, S.Q.; Chen, Y.; Yan, J. Synergistic effect of phosphorus-nitrogen and silicon-containing chain extenders on the mechanical properties, flame retardancy and thermal degradation behavior of waterborne polyurethane. RSC Adv. 2016, 6, 72409–72422. [Google Scholar] [CrossRef]
- Wirasaputra, A.; Zhao, J.; Zhu, Y.; Liu, S.; Yuan, Y. Application of bis(glycidyloxy)phenylphosphine oxide as a chain extender for polyamide-6. RSC Adv. 2015, 5, 31878–31885. [Google Scholar] [CrossRef]
- Zhao, H.B.; Chen, L.; Yang, J.C.; Ge, X.G.; Wang, Y.Z. A novel flame-retardant-free copolyester: Cross-linking towards self extinguishing and non-dripping. J. Mater. Chem. 2012, 22, 19849–19857. [Google Scholar] [CrossRef]
- Zhao, H.B.; Liu, B.W.; Wang, X.L.; Chen, L.; Wang, X.L.; Wang, Y.Z. A flame-retardant-free and thermo-cross-linkable copolyester: Flame-retardant and anti-dripping mode of action. Polymer 2014, 55, 2394–2403. [Google Scholar] [CrossRef]
- Zhao, H.B.; Wang, X.L.; Guan, Y.; Wang, X.L.; Chen, L.; Wang, Y.Z. Block self-cross-linkable poly(ethylene terephthalate) copolyester via solid-state polymerization: Crystallization, cross-linking, and flame retardance. Polymer 2015, 70, 68–76. [Google Scholar] [CrossRef]
- Dong, X.; Chen, L.; Duan, R.T.; Wang, Y.Z. Phenylmaleimide-containing pet-based copolyester: Cross-linking from 2π + π cycloaddition toward flame retardance and anti-dripping. Polym. Chem. 2016, 7, 2698–2708. [Google Scholar] [CrossRef]
- Jing, X.K.; Wang, X.S.; Guo, D.M.; Zhang, Y.; Zhai, F.Y.; Wang, X.L.; Chen, L.; Wang, Y.Z. The high-temperature self-crosslinking contribution of azobenzene groups to the flame retardance and anti-dripping of copolyesters. J. Mater. Chem. A 2013, 1, 9264–9272. [Google Scholar] [CrossRef]
- Wu, J.N.; Chen, L.; Fu, T.; Zhao, H.B.; Guo, D.M.; Wang, X.L.; Wang, Y.Z. New application for aromatic schiff base: High efficient flame-retardant and anti-dripping action for polyesters. Chem. Eng. J. 2018, 336, 622–632. [Google Scholar] [CrossRef]
- Yang, A.-H.; Deng, C.; Chen, H.; Wei, Y.-X.; Wang, Y.-Z. A novel schiff-base polyphosphate ester: Highly-efficient flame retardant for polyurethane elastomer. Polym. Degrad. Stab. 2017, 144, 70–82. [Google Scholar] [CrossRef]
- Naik, A.D.; Fontaine, G.; Bellayer, S.; Bourbigot, S. Crossing the traditional boundaries: Salen-based schiff bases for thermal protective applications. ACS Appl. Mater. Interfaces 2015, 7, 21208–21217. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Q.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Catalyst-free dynamic exchange of aromatic schiff base bonds and its application to self-healing and remolding of crosslinked polymers. J. Mater. Chem. A 2015, 3, 19662–19668. [Google Scholar] [CrossRef]
- Dogan, F.; Kaya, I.; Ocakoglu, K. Synthesis, characterization and thermal degradation kinetics of polyazomethine-ester. J. Indian Chem. Soc. 2008, 85, 1010–1018. [Google Scholar]
- Waltz, J.E.; Taylor, G.B. Determination of molecular weight of nylon. Anal. Chem. 1947, 19, 448–450. [Google Scholar] [CrossRef]
- Foyer, G.; Chanfi, B.H.; Virieux, D.; David, G.; Caillol, S. Aromatic dialdehyde precursors from lignin derivatives for the synthesis of formaldehyde-free and high char yield phenolic resins. Eur. Polym. J. 2016, 77, 65–74. [Google Scholar] [CrossRef]
- Buccella, M.; Dorigato, A.; Pasqualini, E.; Caldara, M.; Fambri, L. Chain extension behavior and thermo-mechanical properties of polyamide 6 chemically modified with 1,1′-carbonyl-bis-caprolactam. Polym. Eng. Sci. 2014, 54, 158–165. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. A review on flame retardant technology in china. Part i: Development of flame retardants. Polym. Adv. Technol. 2009, 21, 1–26. [Google Scholar] [CrossRef]
- Abt, T.; Sanchez-Soto, M.; de Ilarduya, A.M. Toughening of in situ polymerized cyclic butylene terephthalate by chain extension with a bifunctional epoxy resin. Eur. Polym. J. 2012, 48, 163–171. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D.; Pfaendner, R. Influence of accelerated aging on clay-reinforced polyamide 6. Polym. Degrad. Stab. 2009, 94, 389–396. [Google Scholar] [CrossRef]
- Awaja, F.; Daver, F.; Kosior, E.; Cser, F. The effect of chain extension on the thermal behaviour and crystallinity of reactive extruded recycled pet. J. Therm. Anal. Calorim. 2004, 78, 865–884. [Google Scholar] [CrossRef]
- Samyn, F.; Bourbigot, S. Thermal decomposition of flame retarded formulations pa6/aluminum phosphinate/melamine polyphosphate/organomodified clay: Interactions between the constituents? Polym. Degrad. Stab. 2012, 97, 2217–2230. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Jian, R.K.; Wang, Y.Z. Synergistic effect between aluminum hypophosphite and alkyl-substituted phosphinate in flame-retarded polyamide 6. Ind. Eng. Chem. Res. 2013, 52, 17162–17170. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Chen, H.B.; Wang, Y.Z. Aluminum hypophosphite versus alkyl-substituted phosphinate in polyamide 6: Flame retardance, thermal degradation, and pyrolysis behavior. Ind. Eng. Chem. Res. 2013, 52, 2875–2886. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jager, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. e-Polymers 2010, 10, 443–456. [Google Scholar] [CrossRef]
- Huang, W.; He, W.; Long, L.; Yan, W.; He, M.; Qin, S.; Yu, J. Highly efficient flame-retardant glass-fiber-reinforced polyamide 6t system based on a novel dopo-based derivative: Flame retardancy, thermal decomposition, and pyrolysis behavior. Polym. Degrad. Stab. 2018, 148, 26–41. [Google Scholar] [CrossRef]
- Bai, Z.; Lei, S.; Yuan, H.; Gong, X.; Yuen, R.K.K. Investigation on flame retardancy, combustion and prolysis behavior of flame retarded unsaturated polyester resin with a star-shaped phosphorus-containing compound. J. Anal. Appl. Pyrolysis 2014, 105, 317–326. [Google Scholar] [CrossRef]
- Xing, W.; Song, L.; Yuan, H.U.; Zhou, S.; Kun, W.U.; Chen, L. Thermal properties and combustion behaviors of a novel uv-curable flame retarded coating containing silicon and phosphorus. Polym. Degrad. Stab. 2009, 94, 1503–1508. [Google Scholar] [CrossRef]
- Seefeldt, H.; Duemichen, E.; Braun, U. Flame retardancy of glass fiber reinforced high temperature polyamide by use of aluminum diethylphosphinate: Thermal and thermo-oxidative effects. Polym. Int. 2013, 62, 1608–1616. [Google Scholar] [CrossRef]
- Doğan, M.; Bayramlı, E. The flame retardant effect of aluminum phosphinate in combination with zinc borate, borophosphate, and nanoclay in polyamide-6. Fire Mater. 2014, 38, 92–99. [Google Scholar] [CrossRef]











| Samples | Composition (wt.%) | ||
|---|---|---|---|
| PA6 | AlPi | DES | |
| PA6 | 100 | — | — |
| PA6/DES-1 | 99 | — | 1 |
| PA6/DES-2 | 98 | — | 2 |
| PA6/DES-3 | 97 | — | 3 |
| PA6/DES-4 | 96 | — | 4 |
| PA6/DES-5 | 95 | — | 5 |
| PA6/AlPi-13 | 87 | 13 | — |
| PA6/AlPi-16 | 84 | 16 | — |
| PA6/AlPi/DES-0.5 | 86.5 | 13 | 0.5 |
| PA6/AlPi/DES-1 | 86 | 13 | 1 |
| PA6/AlPi/DES-1.5 | 85.5 | 13 | 1.5 |
| PA6/AlPi/DES-2 | 85 | 13 | 2 |
| Samples | Mechanical Properties | Melt Flow Rate [g/(10 min)] | ||
|---|---|---|---|---|
| Izod Notched Impact Strength (kJ/m2) | Tensile Strength (MPa) | Flexural Strength (MPa) | ||
| PA6 | 6.6 ± 0.2 | 59.5 ± 1.0 | 89.8 ± 1.2 | 17.2 ± 0.4 |
| PA6/AlPi-13 | 6.4 ± 0.3 | 56.4 ± 0.9 | 86.1 ± 1.0 | 11.8 ± 0.3 |
| PA6/AlPi-16 | 5.8 ± 0.1 | 55.0 ± 0.9 | 83.8 ± 0.8 | 12.1 ± 0.4 |
| PA6/AlPi/DES-0.5 | 6.9 ± 0.2 | 60.6 ± 1.2 | 90.6 ± 0.7 | 7.2 ± 0.2 |
| PA6/AlPi/DES-1 | 7.2 ± 0.1 | 63.3 ± 1.0 | 93.3 ± 0.9 | 3.5 ± 0.2 |
| PA6/AlPi/DES-1.5 | 7.2 ± 0.3 | 63.9 ± 0.9 | 97.6 ± 1.0 | 2.3 ± 0.2 |
| PA6/AlPi/DES-2 | 7.3 ± 0.2 | 61.9 ± 1.2 | 95.6 ± 1.0 | 2.3 ± 0.1 |
| Samples | Tm (°C) | ΔHm (J/g) | Wf (%) | Xc (%) |
|---|---|---|---|---|
| PA6 | 223.5 | 49.9 | 100 | 26.2 |
| PA6/AlPi-13 | 222.5 | 43.5 | 87 | 26.3 |
| PA6/AlPi-16 | 223.0 | 37.8 | 84 | 23.7 |
| PA6/AlPi/DES-0.5 | 223.0 | 42.3 | 86.5 | 25.8 |
| PA6/AlPi/DES-1 | 223.0 | 40.2 | 86 | 24.6 |
| PA6/AlPi/DES-1.5 | 223.5 | 39.1 | 85.5 | 24.0 |
| PA6/AlPi/DES-2 | 221.9 | 37.3 | 85 | 23.1 |
| Samples | LOI (%) | UL-94 | ||||
|---|---|---|---|---|---|---|
| Thickness (mm) | T12 (s) | T22 (s) | Burning Drips | Rating | ||
| PA6 | 24.2 | 1.6 | >60 | >60 | Yes | NR |
| PA6/DES-2 | 25.5 | 1.6 | >60 | >60 | Yes | NR |
| PA6/AlPi-13 | 29.3 | 0.8 | 42 | >60 | Yes | NR |
| 1.6 | 2 | 2 | Yes | V-2 | ||
| PA6/AlPi-16 | 31.8 | 0.8 | 2 | 5 | Yes | V-2 |
| 1.6 | 1 | 1 | No | V-0 | ||
| PA6/AlPi/DES-0.5 | 32.2 | 0.8 | 10 | 3 | Yes | V-2 |
| 1.6 | 2 | 2 | No | V-0 | ||
| PA6/AlPi/DES-1 | 33.5 | 0.8 | 3 | 4 | Yes | V-2 |
| 1.6 | 1 | 2 | No | V-0 | ||
| PA6/AlPi/DES-1.5 | 34.9 | 0.8 | 2 | 2 | No | V-0 |
| 1.6 | 1 | 1 | No | V-0 | ||
| PA6/AlPi/DES-2 | 35.4 | 0.8 | 3 | 1 | No | V-0 |
| 1.6 | 2 | 1 | No | V-0 | ||
| Samples | TTI (s) | Peak HRR (kW/m2) | THR (MJ/m2) | Peak RSR ((m2/s)/m2) | TSR (m2/m2) | Residue (wt.%) |
|---|---|---|---|---|---|---|
| PA6 | 248 | 759.2 | 121.8 | 1.5 | 144.3 | 3.6 |
| PA6/AlPi-13 | 230 | 536.5 | 123.9 | 9.8 | 1677.1 | 5.1 |
| PA6/AlPi/DES-1.5 | 228 | 478.0 | 126.6 | 7.5 | 1620.3 | 6.7 |
| Samples | Element Content (wt.%) | |||
|---|---|---|---|---|
| C | O | Al | P | |
| PA6 | 81.2 ± 0.8 | 18.8 ± 0.8 | — | — |
| PA6/AlPi-13 | 37.7 ± 1.2 | 40.8 ± 3.1 | 8.6 ± 0.7 | 12.9 ± 1.5 |
| PA6/AlPi/DES-1.5 | 27.2 ± 1.6 | 47.2 ± 1.8 | 9.5 ± 0.6 | 16.0 ± 1.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.; Cai, J.; Liu, S.; Lai, H.; Zhao, J. Chain Extension and Synergistic Flame-Retardant Effect of Aromatic Schiff Base Diepoxide on Polyamide 6/Aluminum Diethylphosphinate Composites. Materials 2019, 12, 2217. https://doi.org/10.3390/ma12142217
Liang T, Cai J, Liu S, Lai H, Zhao J. Chain Extension and Synergistic Flame-Retardant Effect of Aromatic Schiff Base Diepoxide on Polyamide 6/Aluminum Diethylphosphinate Composites. Materials. 2019; 12(14):2217. https://doi.org/10.3390/ma12142217
Chicago/Turabian StyleLiang, Tianxiang, Jianan Cai, Shumei Liu, Hualin Lai, and Jianqing Zhao. 2019. "Chain Extension and Synergistic Flame-Retardant Effect of Aromatic Schiff Base Diepoxide on Polyamide 6/Aluminum Diethylphosphinate Composites" Materials 12, no. 14: 2217. https://doi.org/10.3390/ma12142217
APA StyleLiang, T., Cai, J., Liu, S., Lai, H., & Zhao, J. (2019). Chain Extension and Synergistic Flame-Retardant Effect of Aromatic Schiff Base Diepoxide on Polyamide 6/Aluminum Diethylphosphinate Composites. Materials, 12(14), 2217. https://doi.org/10.3390/ma12142217