The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene-Based Nanomaterial Preparation
2.2. Mineral Glass Optic Microscopy in Polarized Light with Crossed Nicols
2.3. Mechanical Property Evaluation
2.4. Water Absorption
2.5. Scanning Electron Microscopy
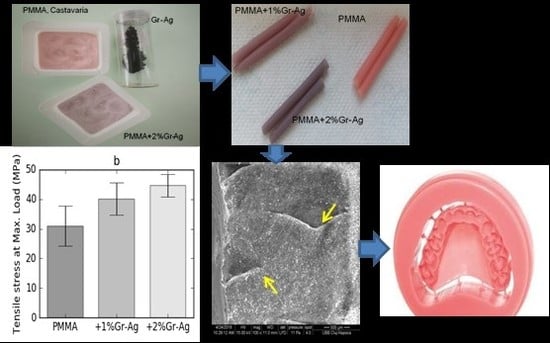
3. Results
3.1. Optical Microscopy in Polarized Light
3.2. SEM Analyses of Fillers
3.3. Compression
3.4. Flexural Strength
3.5. Tensile Strength
3.6. Water Absorption
3.7. SEM Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moldovan, M.; Prodan, D.; Sarosi, C.; Carpa, R.; Socaci, C.; Rosu, M.C.; Pruneanu, S. Synthesis, morpho-structural properties and antibacterial effect of silicate based composites containing graphene oxide/hydroxyapatite. Mater. Chem. Phys. 2018, 217, 48–53. [Google Scholar] [CrossRef]
- Xie, H.; Cao, T.; Rodriguez-Lozano, F.J.; Luong-Van, E.K.; Rosa, V. Graphene for the development of the next-generation of bios for dental and medical applications. Dent. Mater. 2017, 33, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Kamonkhantikul, K.; Arksornnukit, M.; Takahashi, H. Pressure transmission area and maximum pressure transmission of different thermoplastic resin denture base materials under impact load. J. Prosthodont. Res. 2018, 62, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Alhareb, A.O.; MdAkil, H.; Ahmad, Z.A. Impact strength, fracture toughness and hardness improvement of PMMA denture base through addition of nitrile rubber/ceramic fillers. Saudi. J. Dent. Res. 2017, 8, 26–34. [Google Scholar] [CrossRef]
- Alp, G.; Johnston, W.M.; Yilmaz, B. Optical properties and surface roughness of prepolymerized poly(methyl methacrylate) denture base materials. J. Prosthet. Dent. 2019, 121, 347–352. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Baker, C.; Pradham, L.; Pakstis, L.; Darrin, J.; Shah, S.I. Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotech. 2005, 5, 244–249. [Google Scholar] [CrossRef]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef]
- Nisar, S.; Moeen, F.; Khan, Y.H. Effect of curing regimens on physical properties of heat cure acrylic resin. Pakistan Oral Dent. J. 2016, 36, 156–160. [Google Scholar]
- ElBahra, S.; Ludwig, K.; Samran, A. Linear and volumetric dimensional changes of injection-molded PMMA denture base resins. Dent. Mater. 2013, 29, 1091–1097. [Google Scholar] [CrossRef]
- Doğan, A.; Bek, B.; Cevik, N.N.; Usanmaz, A. The effect of preparation conditions of acrylic denture base materials on the level of residual monomer, mechanical properties and water absorption. J. Dent. 1995, 23, 313–318. [Google Scholar] [CrossRef]
- Iwata, Y. Assessment of clasp design and flexural properties of acrylic denture base materials for use in non-metal clasp dentures. J. Prosthodont. Res. 2016, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Nodehi, A.; Atai, M. PMMA/double-modified organoclay nanocomposites as fillers for denture base materials with improved mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 90, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Uyar, T.; Çökeliler, D.; Doğan, M.; Kocum, I.C.; Karatay, O.; Denkbas, E.B. Electrospun nanofiber reinforcement of dental composites with electromagnetic alignment approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 762–770. [Google Scholar] [CrossRef]
- Lee, J.; Jo, J.; Kim, D.; Patel, K.D.; Kim, H.; Lee, H. Nano-graphene oxide incorporated into PMMA resin to prevent microbial adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef] [PubMed]
- Sarosi, C.; Biris, A.R.; Antoniac, A.; Boboia, S.; Alb, C.; Antoniac, I.; Moldovan, M. The nanofiller effect on properties of experimental graphene dental nanocomposites. J. Adhes. Sci. Technol. 2016, 30, 1779–1794. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-based nanomaterials for tissue engineering in the dental field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef]
- Biris, A.R.; Dervishi, E.; Ardelean, S.; Lazar, M.D.; Watanabe, F.; Biris, G.L.; Misan, I.; Biris, A.S. Synthesis of graphite structures decorated with Ag with a new Ag/MgO catalytic system by chemical deposition of radio frequency vapor. Mater. Chem. Phys. 2013, 138, 454–461. [Google Scholar] [CrossRef]
- Sava, S.; Moldovan, M.; Sarosi, C.; Mesaros, A.; Dudea, D.; Alb, C. Effects of graphene addition on the mechanical properties of composites for dental restoration. Mater. Plast. 2015, 23, 90–92. [Google Scholar]
- Khalil, W.M. Measurement of water sorption of five different composite resin materials. J. Bagh Coll. Dent. 2005, 17, 37–41. [Google Scholar]
- Akinci, A.; Sen, S.; Sen, U. Friction and wear behavior of zirconium oxide reinforced PMMA composites. Compos. Part B 2014, 56, 42–47. [Google Scholar] [CrossRef]
- Suteewong, T.; Wongpreecha, J.; Polpanich, D.; Jangpatarapongsa, K.; Kaewsaneha, C.; Tangboriboonrat, P. PMMA particles coated with chitosan-silver nanoparticles as a dual antibacterial modifier for natural rubber latex films. Colloids Surf. B Biointerfaces 2019, 174, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Vallitu, P.K. Dimensional accuracy and stability of polymethyl methacrylate reinforced with metal wire or with continuous glass fiber. J. Prosthet. Dent. 1996, 75, 617–621. [Google Scholar] [CrossRef]
- Lin, F.; Yang, C.; Zeng, Q.H.; Xiang, Y. Morphological and mechanical properties of graphene-reinforced PMMA nanocomposites using a multiscale analysis. Comp. Mater. Sci. 2018, 150, 107–120. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar]
- Vallés, C.; Kinloch, I.A.; Young, R.J.; Wilson Rourke, J.P. Graphene oxide and base-washed graphene oxide as reinforcements in PMMA nanocomposites. Compos. Sci. Technol. 2013, 88, 158–164. [Google Scholar]
- Uddin, E.; Layek, R.K.; Kim, N.H.; Hui, D.; Lee, J.H. Preparation and properties of reduced graphene oxide/polyacrylonitrile nanocomposites using polyvinyl phenol. Compos. Part B 2015, 80, 238–245. [Google Scholar] [CrossRef]
- Puertolas, J.A.; Castro, M.; Morris, J.A.; Rios, R.; Anson-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Saini, P.; Gupta, D.; Choudhary, V. Electrical and mechanical properties of PMMA/reduced graphene oxide nanocomposites prepared via in situ polymerization. J. Mater. Sci. 2013, 48, 6223–6232. [Google Scholar] [CrossRef]
- Layek, R.K.; Uddin, M.E.; Kim, N.H.; Tak Lau, A.K.; Lee, J.H. Noncovalent functionalization of reduced graphene oxide with pluronic F127 and its nanocomposites with gum Arabic. Composites Part B 2017, 128, 155–163. [Google Scholar] [CrossRef]
- Ajaj-AlKordy, N.M.; Alsaadi, M.H. Elastic modulus and flexural strength comparisons of high-impact and traditional denture base acrylic resins. Saudi. Dent. J. 2014, 26, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Shenoy, K.; Ravishankar, K.S. Flexural strength of E-glass-reinforced PMMA. Int. J. Exp. Dent. Sci. 2014, 3, 24–28. [Google Scholar] [CrossRef]
- Ghaffari, T.; Hamedi-Rad, F. Effect of silver nano-particles on the tensile strength of acrylic resins. J. Dent. Clin. Prospect. 2015, 9, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, A.; Sahin, O.; Kurkcuoglu, I.; Dede, D.O.; Ozdemir, T.; Hazer, B. Silver nanoparticle incorporation effect in mechanical and thermal properties of denture base acrylic resin. J. Appl. Oral. Sci. 2016, 24, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Seyrek, M.; Ulusoy, K.G. Biocompatibility of Dental Polymers. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Publisher: Elche, Spain, 2016; pp. 88–98. [Google Scholar]
- Paz, E.; Forriol, F.; del Real, J.C.; Dunne, N. Graphene oxide versus graphene for optimization of PMMA bone cement for orthopaedic applications. Mater Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Segerstrom, S.; Sandborgh-Englund, G.; Ruyter, E.I. Biological and physicochemical properties of carbon-graphite fibre-reinforced polymers intended for implant supra structures. Eur. J. Oral. Sci. 2011, 119, 246–252. [Google Scholar] [CrossRef] [PubMed]








| Sample | Maximum Load (N) | Modulus of Rupture (MPa) |
|---|---|---|
| M | 18.1 ± 4.2 a | 13.4 ± 3.1 a |
| P1 | 53.8 ± 19.2 b | 35.00 ± 8.3 b |
| P2 | 81.3 ± 18.3 c | 36.89 ± 6.7 b |
| Sample | Saliva Exposure Time | Distilled Water Exposure Time | ||
|---|---|---|---|---|
| 7 Days (%) | 28 Days (%) | 7 Days (%) | 28 Days (%) | |
| M | 1.26 ± 0.13 a | 1.44 ± 0.07 a | 1.16 ± 0.19 a | 1.67 ± 0.64 a |
| P1 | 0.78 ± 0.14 b | 1.21 ± 0.10 b | 1.25 ± 0.21 b | 1.65 ± 0.03 b |
| P2 | 0.44 ± 0.10 c | 1.18 ± 0.34 b | 0.65 ± 0.15 c | 1.24 ± 0.15 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. https://doi.org/10.3390/ma12142335
Bacali C, Badea M, Moldovan M, Sarosi C, Nastase V, Baldea I, Chiorean RS, Constantiniuc M. The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials. 2019; 12(14):2335. https://doi.org/10.3390/ma12142335
Chicago/Turabian StyleBacali, Cecilia, Mindra Badea, Marioara Moldovan, Codruta Sarosi, Vivi Nastase, Ioana Baldea, Radu Stefan Chiorean, and Mariana Constantiniuc. 2019. "The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins" Materials 12, no. 14: 2335. https://doi.org/10.3390/ma12142335
APA StyleBacali, C., Badea, M., Moldovan, M., Sarosi, C., Nastase, V., Baldea, I., Chiorean, R. S., & Constantiniuc, M. (2019). The Influence of Graphene in Improvement of Physico-Mechanical Properties in PMMA Denture Base Resins. Materials, 12(14), 2335. https://doi.org/10.3390/ma12142335