Surface Modification of Polycarbonate by an Atmospheric Pressure Argon Microwave Plasma Sheet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Investigation Methodology
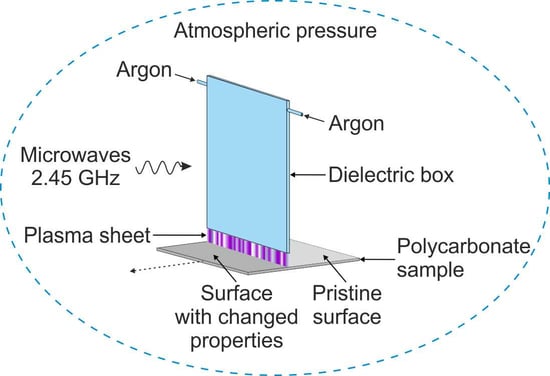
2.3. Polycarbonate Material Surface Modification by an Argon Microwave Plasma
2.4. Contact Angle Goniometry
2.5. Atomic Force Microscopy (AFM)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De la Colina-Martínez, A.L.; Martínez-Barrera, G.; Barrera-Díaz, C.E.; Ávila-Córdoba, L.I.; Ureña-Núñez, F.; Delgado-Hernández, D.J. Recycled polycarbonate from electronic waste and its use in concrete: Effect of irradiation. Constr. Build. Mater. 2019, 201, 778–785. [Google Scholar] [CrossRef]
- Kamps, J.H.; Scheffler, C.; Simon, F.; van der Heijden, R.; Verghese, N. Functional polycarbonates for improved adhesion to carbon fibre. Compos. Sci. Tech. 2018, 167, 448–455. [Google Scholar] [CrossRef]
- Mishraa, V.; Sharma, R.; Khatri, N.; Garg, H.; Karar, V.; Khan, G.S.; Sarepaka, R.V. Processing of polycarbonate by ultra-precision machining for optical applications. Mater. Today Proc. 2018, 5, 25130–25138. [Google Scholar] [CrossRef]
- Larosa, C.; Stura, E.; Eggenhöffner, R.; Nicolini, C. Optimization of optical properties of polycarbonate film with thiol gold-nanoparticles. Materials 2009, 2, 1193–1204. [Google Scholar] [CrossRef]
- Barletta, M.; Puopolo, M.; Rubino, G.; Tagliaferri, V.; Vesco, S. Hard transparent coatings on thermoplastic polycarbonate. Prog. Org. Coat. 2016, 90, 178–186. [Google Scholar] [CrossRef]
- Qureshi, A.; Shah, S.; Pelagade, S.; Singh, N.L.; Mukherjee, S.; Tripathi, A.; Despande, U.P.; Shripathi, T. Surface modification of polycarbonate by plasma treatment. J. Phys. Conf. Ser. 2010, 208, 012108. [Google Scholar] [CrossRef]
- Soliman, E.A.; Samir, A.; Hassan, A.M.A.; Mohy-Eldin, M.S.; El-Naim, G.A. Limiting the migration of bisphenol A from polycarbonate using Dielectric Barrier Discharge. Open J. Synth. Theory Appl. 2014, 3, 27–36. [Google Scholar] [CrossRef]
- Rogalsky, S.P.; Moshynets, O.V.; Lyoshina, L.G.; Tarasyuk, O.P. Antimicrobial polycarbonates for biomedicalapplications. EPMA J. 2014, 5 (Suppl. S1), A133. [Google Scholar] [CrossRef]
- Harper, C. Handbook of Plastics, Elastomers and Composites, 2nd ed.; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Guenter, K.H. Coating of plastics-coatings on plastics. Proc. SPIE 1998, 896, 134–139. [Google Scholar] [CrossRef]
- Kruse, A.; Krüger, G.; Baalmann, A.; Hennemann, O.-D. Surface pretreatment of plastics for adhesive bonding. J. Adhes. Sci. Technol. 1995, 9, 1611–1621. [Google Scholar] [CrossRef]
- Strobel, M.; Branch, M.C.; Ulsh, M.; Kapaun, R.S.; Kirk, S.; Lyons, C.S. Flame surface modification of polypropylene film. J. Adhes. Sci. Technol. 1996, 10, 515–539. [Google Scholar] [CrossRef]
- Kraus, E.; Baudrit, B.; Heidemeyer, P.; Bastian, M.; Stoyanov, O.; Starostina, I. Surface treatment with ultraviolet laser for adhesive bonding of polymeric materials. J. Adhes. 2017, 93, 204–215. [Google Scholar] [CrossRef]
- Walzak, M.J.; Flynn, S.; Foerch, R.; Hill, J.M.; Karbashewski, E.; Lin, A.; Strobel, M. UV and ozone treatment of polypropylene and poly (ethylene terephthalate). J. Adhes. Sci Technol. 1995, 9, 1229–1248. [Google Scholar] [CrossRef]
- López-Santos, C.; Yubero, F.; Cotrino, J.; Barranco, A.; González-Elipe, A.R. Plasmas and atom beam activation of the surface of polymers. J. Phys. D Appl. Phys. 2008, 41, 225209. [Google Scholar] [CrossRef]
- Chan, C.-M.; Ko, T.-M.; Hiraoka, H. Polymer surface modification by plasmas and photons. Surf. Sci. Rep. 1996, 24, 1–54. [Google Scholar] [CrossRef]
- Gupta, B.; Hilborn, J.; Hollenstein, C.; Plummer, C.J.G.; Houriet, R.; Xanthopoulos, N. Surface modification of polyester films by RF plasma. J. Appl. Polym. Sci. 2000, 78, 1083–1091. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef] [Green Version]
- Hidzir, N.M.; Hill, D.J.T.; Taran, E.; Martin, D.; Grøndahl, L. Argon plasma treatment-induced grafting of acrylic acid onto expanded poly(tetrafluoroethylene) membranes. Polymer 2013, 54, 6536–6546. [Google Scholar] [CrossRef]
- López-García, J.; Cupessala, F.; Humpolíček, P.; Lehocký, M. Physical and morphological changes of poly (tetrafluoroethylene) after using non-thermal plasma-treatments. Materials 2018, 11, 2013. [Google Scholar] [CrossRef]
- Kolská, Z.; Řezníčková, A.; Hnatowicz, V.; Švorčík, V. PTFE surface modification by Ar plasma and its characterization. Vacuum 2012, 86, 643–647. [Google Scholar] [CrossRef]
- Chien, H.-H.; Ma, K.-J.; Kuo, C.-H.; Huang, S.-W. Effects of plasma power and reaction gases on the surface properties of ePTFE materials during a plasma modification process. Surf. Coat. Technol. 2013, 228, S477–S481. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Chang, K.-H.; Hung, T.-S.; Chen, K.-S.; Inagaki, N. Atmospheric-pressure plasma treatment on polystyrene for the photo-induced grafting polymerization of N-isopropylacrylamide. Thin Solid Films 2010, 518, 7568–7573. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Reniers, F. Plasma-modified polymer surfaces: Characterization using XPS. J. Electron Spectrosc. Relat. Phenom. 2010, 178–179, 394–408. [Google Scholar] [CrossRef]
- Hrycak, B.; Sikora, A.; Moczała, M.; Czylkowski, D.; Jasiński, M.; Dors, M. Atmospheric pressure microwave argon plasma sheet for wettability modification of polyethylene surfaces. IEEE Trans. Plasma Sci. 2019, 47, 1309–1315. [Google Scholar] [CrossRef]
- Sowe, M.; Novák, L.; Vesel, A.; Junkar, I.; Lehock, M.; Sáha, P.; Chodák, I. Analysis and characterization of printed plasma-treated polyvinyl chloride. Int. J. Polym. Anal. Charact. 2009, 14, 641–651. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Vukušić, T.; Vesel, A.; Holc, M.; Ščetar, M.; Jambrak, A.R.; Mozetič, M. Modification of physico-chemical properties of acryl-coated polypropylene foils for food packaging by reactive particles from oxygen plasma. Materials 2018, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Šourková, H.; Primc, G.; Špatenka, P. Surface functionalization of polyethylene granules by treatment with low-pressure air plasma. Materials 2018, 11, 885. [Google Scholar] [CrossRef] [PubMed]
- Mandolfino, C.; Lertora, E.; Gambaro, C.; Pizzorni, M. Functionalization of neutral polypropylene by using low pressure plasma treatment: Effects on surface characteristics and adhesion properties. Polymers 2019, 11, 202. [Google Scholar] [CrossRef]
- Zajíčková, L.; Buršıková, V.; Peřina, V.; Macková, A.; Subedi, D.; Janča, J.; Smirnov, S. Plasma modification of polycarbonates. Surf. Coat. Technol. 2001, 142–144, 449–454. [Google Scholar] [CrossRef]
- Larsson, A.; Dérand, H. Stability of polycarbonate and polystyrene surfaces after hydrophilization with high intensity oxygen RF plasma. J. Colloid Interface Sci. 2002, 246, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, A.; Bulkin, P.; Drevillon, B. Plasma treatment of polycarbonate for improved adhesion. J. Vac. Sci. Technol. A Vac. Surf. Films 2002, 20, 245–250. [Google Scholar] [CrossRef]
- Kitova, S.; Minchev, M.; Danev, G. RF plasma treatment of polycarbonate substrates. J. Optoelectron. Adv. Mater. 2005, 7, 2607–2612. [Google Scholar]
- Sharma, R.; Holcomb, E.; Trigwell, S.; Mazumder, M. Stability of atmospheric-pressure plasma induced changes on polycarbonate surfaces. J. Electrost. 2007, 65, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Subedi, D.P.; Madhup, D.K.; Adhikari, K.; Joshi, U.M. Plasma treatment at low pressure for the enhancement of wettability of polycarbonate. Indian J. Pure Appl. Phys. 2008, 46, 540–544. [Google Scholar]
- Pedrosa, P.; Chappé, J.-M.; Fonseca, C.; Machado, A.V.; Nóbrega, J.M.; Vaz, F. Plasma surface modification of polycarbonate and poly (propylene) substrates for biomedical electrodes. Plasma Process. Polym. 2010, 7, 676–686. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.A.; Mekala, M.; Yoganand, C.P.; Navaneetha Pandiyaraj, K. Studies on modification of surface properties in polycarbonate (PC) film induced by DC glow discharge plasma. Int. J. Polym. Sci. 2011, 2011, 426057. [Google Scholar] [CrossRef]
- De Vietroa, N.; Belforte, L.; Lambertini, V.G.; Fracassi, F. Low pressure plasma modified polycarbonate: A transparent, low reflective and scratch resistant material for automotive applications. Appl. Surf. Sci. 2014, 307, 698–703. [Google Scholar] [CrossRef]
- Kelar, J.; Shekargoftar, M.; Krumpolec, R.; Homola, T. Activation of polycarbonate (PC) surfaces by atmospheric pressure plasma in ambient air. Polym. Test. 2018, 67, 428–434. [Google Scholar] [CrossRef]
- Jeon, B.J. Control of optical properties by the stepwise chemical and plasma spray treatment of polycarbonate. Appl. Sci. Converg. Technol. 2018, 27, 135–139. [Google Scholar] [CrossRef]
- Ul Haq, A.; Boyd, A.; Acheson, J.; McLaughlin, J.; Meenan, B.J. Corona discharge-induced functional surfaces of polycarbonate and cyclic olefins substrates. Surf. Coat. Technol. 2019, 362, 185–190. [Google Scholar] [CrossRef]
- Dewez, J.-L.; Deren, A.; Rouxhet, P.G.; Schneider, Y.J.; Legras, R. Surface study of polycarbonate membranes used as a substratum for animal cell culture. Surf. Interface Anal. 1991, 17, 499–502. [Google Scholar] [CrossRef]
- Nowakowska, H.; Czylkowski, D.; Hrycak, B.; Jasiński, M. Characterization of a novel microwave plasma sheet source operated at atmospheric pressure. Plasma Sour. Sci. Technol. 2018, 27, 085008. [Google Scholar] [CrossRef]
- Jasiński, M.; Mizeraczyk, J. Plasma sheet generated by microwave discharge at atmospheric pressure. IEEE Trans. Plasma Sci. 2011, 39, 2136–2137. [Google Scholar] [CrossRef]
- Czylkowski, D.; Hrycak, B.; Jasiński, M. Compact microwave plasma device for surface treatment. Przegląd Elektrotechniczny 2018, 94, 30–33. [Google Scholar] [CrossRef]
- Sikora, A.; Tomczuk, K. Impact of the LED-based light source working regime on the degradation of polymethyl methacrylate. Lighting Res. Technol. 2019. [Google Scholar] [CrossRef]
- Sikora, A.; Janus, P.; Sierakowski, A. The impact of the light exposure on the morphological properties of selected photoresists. Optica Appl. 2019, 49, 177–185. [Google Scholar] [CrossRef]
- Robertson, C.; Wertheimer, M.; Fournier, D.; Lamarre, L. Study on the morphology of XLPE power cable by means of atomic force microscopy. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 283–288. [Google Scholar] [CrossRef]
- Sikora, A.; Bednarz, Ł.; Fałat, T.; Wałecki, M.; Adamowska, M. The investigation of the simulated solar radiation impact on the micro- and nanoscale morphology and mechanical properties of the sheet moulded composite surface. Mater. Sci. Poland 2016, 34, 641–649. [Google Scholar] [CrossRef]
- Lochyński, P.; Sikora, A.; Szczygieł, B. Surface morphology and passive film composition after pickling and electropolishing. Surf. Eng. 2017, 33, 395–403. [Google Scholar] [CrossRef]
- Nowicki, M.; Richter, A.; Wolf, B.; Kaczmarek, H. Nanoscale mechanical properties of polymers irradiated by UV. Polymer 2003, 44, 6599–6606. [Google Scholar] [CrossRef]
- Moisan, M.; Beaudry, C.; Lepprince, P. A new HF device or the production of long plasma columns at a high electron density. Phys. Lett. A 1974, 50, 125–126. [Google Scholar] [CrossRef]
- Moisan, M.; Zakrzewski, Z.; Pantel, R.; Leprince, P. A waveguide-based launcher to sustain long plasma columns through the propagation of an electromagnetic surface wave. IEEE Trans. Plasma Sci. 1984, 12, 203–214. [Google Scholar] [CrossRef]
- Hnilica, J.; Potočňáková, L.; Stupavská, M.; Kudrle, V. Rapid surface treatment of polyamide 12 by microwave plasma jet. Appl. Surf. Sci. 2014, 288, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Hnilica, J.; Kudrle, V.; Potočňáková, L. Surface treatment by atmospheric-pressure surfatron jet. IEEE Trans. Plasma Sci. 2012, 40, 2925–2930. [Google Scholar] [CrossRef]
- Moisan, M.; Zakrzewski, Z.; Rostaing, J.C. Waveguidebased single and multiple nozzle plasma torches: The TIAGO concept. Plasma Sour. Sci. Technol. 2001, 10, 387–394. [Google Scholar] [CrossRef]
- Shin, D.H.; Bang, C.U.; Kim, J.H.; Hong, Y.C.; Uhm, H.S.; Park, D.K.; Kim, K.H. Treatment of metal surface by atmospheric microwave plasma jet. IEEE Trans. Plasma Sci. 2006, 34, 1241–1246. [Google Scholar] [CrossRef]
- Ricardo, G.; Rubén, P. Dynamic atomic force microscopy methods. Surf. Sci. Rep. 2002, 47, 197–301. [Google Scholar] [CrossRef]
- Image Metrology. Available online: https://www.imagemet.com/products/spip/ (accessed on 10 September 2017).
- Butt, H.-J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef] [Green Version]
- Sikora, A.; Grabarek, A.; Moroń, L.; Wałecki, M.; Kryla, P. The investigation of the light radiation caused polyethylene based materials deterioration by means of atomic force microscopy. IOP Conf. Ser. Mater. Sci. Eng. 2016, 113, 012016. [Google Scholar] [CrossRef]
- Zajíčková, L.; Subedi, D.P.; Buršıková, V.; Veltruská, K. Study of argon plasma treatment of polycarbonate substrate and its effect on film deposition. Acta Phys. Slovaca 2003, 53, 489–504. [Google Scholar]
- Paynter, R.W. XPS studies of the ageing of plasma-treated polimer surfaces. Surf. Interface Anal. 2000, 29, 56–64. [Google Scholar] [CrossRef]
- Vallon, S.; Drévillon, B.; Poncin-Epaillard, F.; Klemberg-Sapieha, J.E.; Martinu, L. Argon plasma treatment of polycarbonate: In situ spectroellipsometry study and polymer characterizations. J. Vac. Sci. Technol. A 1996, 14, 3194–3201. [Google Scholar] [CrossRef]
- Kasih, T.P. Development of non-thermal atmospheric pressure plasma system for surface modification of polymeric materials. J. Phys. Conf. Ser. 2017, 817, 012069. [Google Scholar] [CrossRef] [Green Version]
- Seidel, C.; Kopf, H.; Gotsmann, B.; Vieth, T.; Fuchs, H.; Reihs, K. Ar plasma treated and Al metallised polycarbonate: A XPS, mass spectroscopy and SFM study. Appl. Surf. Sci. 1999, 150, 19–33. [Google Scholar] [CrossRef]
- Vallon, S.; Hofrichter, A.; Guyot, L.; Drévillon, B.; Klemberg-Sapieha, J.E.; Martinu, L.; Poncin-Epaillard, F. Adhesion mechanisms of silica layers on plasma-treated polymers. Part, I. Polycarbonate. J. Adhes. Sci. Technol. 1996, 10, 1287–1311. [Google Scholar] [CrossRef]
- Byun, T.J.; Shin, K.S.; Kim, Y.J.; Han, J.G. Polycarbonate surface treatment by using an inductively-coupled plasma. J. Korean Phys. Soc. 2009, 55, 1785–1789. [Google Scholar] [CrossRef]
- Subedi, D.P.; Zajíčková, L.; Buršıková, V.; Janca, J. Surface modification of polycarbonate (bisphenol A) by low pressure rf plasma. Himal. J. Sci. 2003, 1, 115–118. [Google Scholar] [CrossRef]







| Plasma Type | Plasma Forming Gas | Pressure | Sample Material | Water Contact Angle of Pristine Sample | Water Contact Angle After Plasma Treatment | Reference |
|---|---|---|---|---|---|---|
| DC plasma | Air | Low | Polytetrafluoroethylene | 108.9° | 74.2° | [20] |
| RF plasma | Air | Low | Polytetrafluoroethylene | 108.9° | 75.2° | [20] |
| Microwave plasma sheet | Argon | Atmospheric | Polyethylene | 79.7° | 29° | [25] |
| Glow discharge | Air | Low | Polypropylene | 97.1° | 32° | [30] |
| Glow discharge | Oxygen | Low | Polypropylene | 97.1° | 14° | [30] |
| DBD | Air | Atmospheric | Polycarbonate | 81.5° | 37.9° | [40] |
| Gliding arc | Air | Atmospheric | Polycarbonate | 81.5° | 30.4° | [40] |
| Corona discharge | Air | Atmospheric | Polycarbonate | 80° | 38° | [42] |
| Corona discharge | Air | Atmospheric | Cyclic olefin copolymer | 96° | 39° | [42] |
| Corona discharge | Air | Atmospheric | Cyclic olefinpolymer | 93° | 27° | [42] |
| Arc plasma | Argon | Atmospheric | Polyethylene | 90° | 38.5° | [66] |
| Microwave plasma sheet | Argon | Atmospheric | Polycarbonate | 76° | 32° | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czylkowski, D.; Hrycak, B.; Sikora, A.; Moczała-Dusanowska, M.; Dors, M.; Jasiński, M. Surface Modification of Polycarbonate by an Atmospheric Pressure Argon Microwave Plasma Sheet. Materials 2019, 12, 2418. https://doi.org/10.3390/ma12152418
Czylkowski D, Hrycak B, Sikora A, Moczała-Dusanowska M, Dors M, Jasiński M. Surface Modification of Polycarbonate by an Atmospheric Pressure Argon Microwave Plasma Sheet. Materials. 2019; 12(15):2418. https://doi.org/10.3390/ma12152418
Chicago/Turabian StyleCzylkowski, Dariusz, Bartosz Hrycak, Andrzej Sikora, Magdalena Moczała-Dusanowska, Mirosław Dors, and Mariusz Jasiński. 2019. "Surface Modification of Polycarbonate by an Atmospheric Pressure Argon Microwave Plasma Sheet" Materials 12, no. 15: 2418. https://doi.org/10.3390/ma12152418
APA StyleCzylkowski, D., Hrycak, B., Sikora, A., Moczała-Dusanowska, M., Dors, M., & Jasiński, M. (2019). Surface Modification of Polycarbonate by an Atmospheric Pressure Argon Microwave Plasma Sheet. Materials, 12(15), 2418. https://doi.org/10.3390/ma12152418