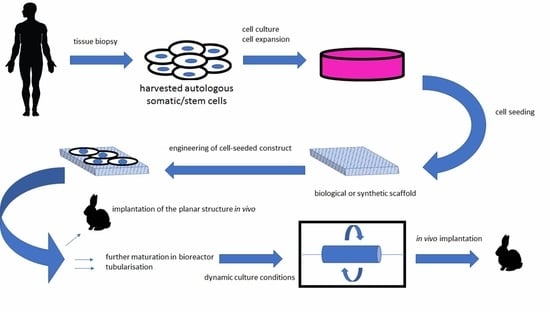
Bioengineered Scaffolds as Substitutes for Grafts for Urethra Reconstruction
Abstract
:1. Introduction
2. Biological Scaffolds
2.1. Acellular Matrices
2.2. Cell Loaded Matrices
3. Biodegradable Synthetic Scaffolds
4. Electrospinning
5. Cell Sheet Engineering
6. Bioprinting
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Shih, E.M.; Graham, J.M. Review of genetic and environmental factors leading to hypospadias. Eur. J. Med. Genet. 2014, 57, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Alwaal, A.; Blaschko, S.D.; McAninch, J.W.; Breyer, B.N. Epidemiology of urethral strictures. Transl. Androl. Urol. 2014, 3, 209–213. [Google Scholar] [PubMed]
- Krishnan, N.R.; Kasthuri, A.S. Iatrogenic Disorders. Med. J. Armed. Forces India. 2005, 61, 2. [Google Scholar] [CrossRef]
- Smith, T.G. Current management of urethral stricture disease. Indian J. Urol. 2016, 32, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.F.; Bhatt, N.R.; MacCraith, E.; Flood, H.D.; Mooney, R.; Leonard, G.; Walsh, M.T. Long-term outcomes of urethral catheterisation injuries: A prospective multi-institutional study. World J. Urol. 2019, 1–8. [Google Scholar] [CrossRef]
- Cheng, L.; Li, S.; Wang, Z.; Huang, B.; Lin, J. A brief review on anterior urethral strictures. Asian J. Urol. 2018, 5, 88–93. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Song, L.-J.; Wang, K.-J.; Lin, J.; Sun, G.; Yue, Z.-J.; Jiang, H.; Shan, Y.-X.; Zhu, S.-X.; Wang, Y.-J.; et al. Changing trends in the causes and management of male urethral stricture disease in China: an observational descriptive study from 13 centres. BJU Int. 2015, 116, 938–944. [Google Scholar] [CrossRef]
- Atala, A. Engineering organs. Curr. Opin. Biotechnol. 2009, 20, 575–592. [Google Scholar] [CrossRef]
- Cui, T.; Terlecki, R.; Atala, A. Tissue engineering in urethral reconstruction. Arch. espanoles de Urol. 2014, 67, 29–34. [Google Scholar]
- Simaioforidis, V.; De Jonge, P.; Sloff, M.; Oosterwijk, E.; Geutjes, P.; Feitz, W.F. Ureteral Tissue Engineering: Where Are We and How to Proceed? Tissue Eng. Part B Rev. 2013, 19, 413–419. [Google Scholar] [CrossRef]
- Dorin, R.P.; Pohl, H.G.; De Filippo, R.E.; Yoo, J.J.; Atala, A. Tubularized urethral replacement with unseeded matrices: What is the maximum distance for normal tissue regeneration? World J. Urol. 2008, 26, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.F.; Cunnane, E.M.; O’Brien, F.J.; Mulvihill, J.J.; Walsh, M.T. Tissue engineered extracellular matrices (ECMs) in urology: Evolution and future directions. Surgeon 2018, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Matoka, D.J.; Cheng, E.Y. Tissue engineering in urology. Can. Urol. Assoc. J. 2009, 3, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Alkildani, S.; Retnasingh, S.; Barbeck, M. Bioprinting of tissue engineering scaffolds. J. Tissue Eng. 2018, 9, 204173141880209. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lin, H.-K.; Frimberger, D.; Epstein, R.B.; Kropp, B.P. Growth of bone marrow stromal cells on small intestinal submucosa: An alternative cell source for tissue engineered bladder. BJU Int. 2005, 96, 1120–1125. [Google Scholar] [CrossRef]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef]
- Fossum, M.; Nordenskjöld, A. Tissue-Engineered Transplants for the Treatment of Severe Hypospadias. Horm. Res. Paediatr. 2010, 73, 148–152. [Google Scholar] [CrossRef]
- Bhargava, S.; Patterson, J.M.; Inman, R.D.; MacNeil, S.; Chapple, C.R. Tissue-Engineered Buccal Mucosa Urethroplasty—Clinical Outcomes. Eur. Urol. 2008, 53, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.O.; Yalcin, H.C.; Pennisi, C.P. From Acellular Matrices to Smart Polymers: Degradable Scaffolds that are Transforming the Shape of Urethral Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 1763. [Google Scholar] [CrossRef] [PubMed]
- Ramuta, T.Ž.; Kreft, M.E. Human Amniotic Membrane and Amniotic Membrane–Derived Cells: How Far Are We from Their Use in Regenerative and Reconstructive Urology? Cell Transplant. 2018, 27, 77–92. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, R.E.; Kornitzer, B.S.; Yoo, J.J.; Atala, A. Penile urethra replacement with autologous cell-seeded tubularized collagen matrices: Collagen matrices for urethral replacement. J. Tissue Eng. Regen. Med. 2015, 9, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Orabi, H.; AbouShwareb, T.; Zhang, Y.; Yoo, J.J.; Atala, A. Cell-Seeded Tubularized Scaffolds for Reconstruction of Long Urethral Defects: A Preclinical Study. Eur. Urol. 2013, 63, 531–538. [Google Scholar] [CrossRef]
- Jia, W.; Tang, H.; Wu, J.; Hou, X.; Chen, B.; Chen, W.; Zhao, Y.; Shi, C.; Zhou, F.; Yu, W.; et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015, 69, 45–55. [Google Scholar] [CrossRef]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.-M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef]
- Larsson, H.M.; Vythilingam, G.; Pinnagoda, K.; Vardar, E.; Engelhardt, E.M.; Sothilingam, S.; Thambidorai, R.C.; Kamarul, T.; Hubbell, J.A.; Frey, P. Fiber density of collagen grafts impacts rabbit urethral regeneration. Sci. Rep. 2018, 8, 10057. [Google Scholar] [CrossRef] [Green Version]
- El-Tabey, N.; Shokeir, A.; Barakat, N.; El-Refaie, H.; El-Hamid, M.A.; Gabr, M. Cell-seeded tubular acellular matrix for replacing a long circumferential urethral defect in a canine model: Is it clinically applicable? Arab. J. Urol. 2012, 10, 192–198. [Google Scholar] [CrossRef]
- Micol, L.A.; Da Silva, L.F.A.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.A.; Feitz, W.F.; Frey, P. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, F.; He, F.; Tian, X.; Tang, S.; Chen, X. A tubular gelatin scaffold capable of the time-dependent controlled release of epidermal growth factor and mitomycin C. Colloids Surfaces B Biointerfaces 2015, 135, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Deng, C.-L.; Liu, W.; Cao, Y.-L. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007, 99, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Versteegden, L.R.; Van Kampen, K.A.; Janke, H.P.; Tiemessen, D.M.; Hoogenkamp, H.R.; Hafmans, T.G.; Roozen, E.A.; Lomme, R.M.; Van Goor, H.; Oosterwijk, E.; et al. Tubular collagen scaffolds with radial elasticity for hollow organ regeneration. Acta Biomater. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Versteegden, L.R.; Ter Meer, M.; Lomme, R.M.; Van Der Vliet, J.A.; Kool, L.J.S.; Van Kuppevelt, T.H.; Daamen, W.F. Self-expandable tubular collagen implants. J. Tissue Eng. Regen. Med. 2018, 12, 1494–1498. [Google Scholar] [CrossRef]
- Sack, B.S.; Mauney, J.R.; Estrada, C.R. Silk Fibroin Scaffolds for Urologic Tissue Engineering. Curr. Urol. Rep. 2016, 17, 16. [Google Scholar] [CrossRef]
- Lv, X.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Peng, X.; Yang, R.; Wang, H.; Xu, Y.; Feng, C. Structural and functional evaluation of oxygenating keratin/silk fibroin scaffold and initial assessment of their potential for urethral tissue engineering. Biomaterials 2016, 84, 99–110. [Google Scholar] [CrossRef]
- Dorati, R.; Colonna, C.; Tomasi, C.; Genta, I.; Bruni, G.; Conti, B. Design of 3D scaffolds for tissue engineering testing a tough polylactide-based graft copolymer. Mater. Sci. Eng. C 2014, 34, 130–139. [Google Scholar] [CrossRef]
- Ellä, V.; Annala, T.; Länsman, S.; Nurminen, M.; Kellomäki, M. Knitted polylactide 96/4 L/D structures and scaffolds for tissue engineering: shelf life, in vitro and in vivo studies. Biomatter 2011, 1, 102–113. [Google Scholar] [CrossRef]
- Selim, M.; Bullock, A.J.; Blackwood, K.A.; Chapple, C.R.; MacNeil, S. Developing biodegradable scaffolds for tissue engineering of the urethra: Biodegradable scaffolds for tissue engineering of the urethra. BJU Int. 2011, 107, 296–302. [Google Scholar] [CrossRef]
- Guelcher, S.A. Biodegradable Polyurethanes: Synthesis and Applications in Regenerative Medicine. Tissue Eng. Part B Rev. 2008, 14, 3–17. [Google Scholar] [CrossRef]
- Hicks, B.G.; Lopez, E.A.; Eastman, R., Jr.; Simonovsky, F.I.; Ratner, B.D.; Wessells, H.; Voelzke, B.B.; Bassuk, J.A. Differential affinity of vitronectin versus collagen for synthetic biodegradable scaffolds for urethroplastic applications. Biomaterials 2011, 32, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Zhang, X.; Yang, L.; Chiellini, F.; Sun, X.; Chiellini, E. Nano/microfibrous polymeric constructs loaded with bioactive agents and designed for tissue engineering applications: A review: Nano/Microfibrous Polymeric Constructs. J. Biomed. Mater. Res. 2014, 102, 1562–1579. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.-L.; Leite, S.M.; A Tamada, J.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly(dl-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Vaquette, C.; Frochot, C.; Rahouadj, R.; Wang, X. An innovative method to obtain porous PLLA scaffolds with highly spherical and interconnected pores. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.; Ponticiello, M.; Leong, K. Fabrication of Controlled Release Biodegradable Foams by Phase Separation. Tissue Eng. 1995, 1, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Ryu, W.H.; Vyakarnam, M.; Greco, R.S.; Prinz, F.B.; Fasching, R.J. Fabrication of multi-layered biodegradable drug delivery device based on micro-structuring of PLGA polymers. Biomed. Microdevices 2007, 9, 845–853. [Google Scholar] [CrossRef]
- Villarreal-Gómez, L.J.; Cornejo-Bravo, J.M.; Vera-Graziano, R.; Grande, D. Electrospinning as a powerful technique for biomedical applications: A critically selected survey. J. Biomater. Sci. Polym. Ed. 2016, 27, 157–176. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, X.; Zhao, W.; Niu, G.; Mo, X.; Fu, Q. Application of Wnt Pathway Inhibitor Delivering Scaffold for Inhibiting Fibrosis in Urethra Strictures: In Vitro and in Vivo Study. IJMS 2015, 16, 27659–27676. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Guo, Q.; Han, F.; Chen, C.; Ling, C.; Chen, W.; Li, B. Electrospun Poly(l-lactide)/Poly(ethylene glycol) Scaffolds Seeded with Human Amniotic Mesenchymal Stem Cells for Urethral Epithelium Repair. Int. J. Mol. Sci. 2016, 17, 1262. [Google Scholar] [CrossRef]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Li, C.; Fu, Q.; Xu, Y.; Li, H. Preparation of PCL/silk fibroin/collagen electrospun fiber for urethral reconstruction. Int. Urol. Nephrol. 2015, 47, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, R.; Zou, Q.; Zhang, K.; Yin, T.; Zhao, W.; Shapter, J.G.; Gao, G.; Fu, Q. Fabrication of Tissue-Engineered Bionic Urethra Using Cell Sheet Technology and Labeling By Ultrasmall Superparamagnetic Iron Oxide for Full-Thickness Urethral Reconstruction. Theranostics 2017, 7, 2509–2523. [Google Scholar] [CrossRef] [PubMed]
- Cattan, V.; Bernard, G.; Rousseau, A.; Bouhout, S.; Chabaud, S.; Auger, F.A.; Bolduc, S. Mechanical Stimuli-induced Urothelial Differentiation in a Human Tissue-engineered Tubular Genitourinary Graft. Eur. Urol. 2011, 60, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, Y.-J.; Kim, C.-H. 3D Bioprinting Technologies for Tissue Engineering Applications. In Genome Editing; Springer Science and Business Media LLC: Berlin, Germany, 2018; Volume 1078, pp. 15–28. [Google Scholar]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar] [Green Version]
- Balogová, A.F.; Hudák, R.; Tóth, T.; Schnitzer, M.; Feranc, J.; Bakoš, D.; Živčák, J. Determination of geometrical and viscoelastic properties of PLA/PHB samples made by additive manufacturing for urethral substitution. J. Biotechnol. 2018, 284, 123–130. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Pi, Q.; Maharjan, S.; Yan, X.; Liu, X.; Singh, B.; Van Genderen, A.M.; Robledo-Padilla, F.; Parra-Saldivar, R.; Hu, N.; Jia, W.; et al. Digitally Tunable Microfluidic Bioprinting of Multilayered Cannular Tissues. Adv. Mater. 2018, 30, 1706913. [Google Scholar] [CrossRef]

| Material | Results | Reference |
|---|---|---|
| human amniotic membrane | confirmed potential, concern about the lack of standardized preparation protocol, storage, and mechanical properties | [22] |
| tubular gelatin scaffold loaded with EGF and MMC | inhibitory potential of scar formation | [31] |
| seeded bladder submucosa | successful repair of a long urethral defect in a canine model | [24] |
| unseeded bladder submucosa | ability to repair short (0.5 cm) urethral defects; long defects (up to 3 cm) were not repaired, increased deposition of collagen and fibrosis detected | [25] |
| collagen scaffold loaded with CB-VEGF | better epithelization, revascularization and smooth muscle regeneration detected | [26] |
| double-layered high-density collagen gel tubes | the regenerative potential of gel tubes observed (animal model); however, 20% of animals developed complications | [27] |
| silk fibroin | good biodegradation properties | [35] |
| modified silk fibroin/keratin films with oxygen-generating substance and calcium peroxide | observed enhanced regenerative potential | [36] |
| Material | Results | Reference |
|---|---|---|
| the graft copolymer of PLA | better mechanical properties when compared to PLA homopolymer | [37] |
| PLGA | autologous tissue-engineered urethras applied in 5 boys remained functional up to 6 years’ follow-up | [16] |
| PLGA | observed potential for tissue engineering of buccal mucosa for urethral repair application | [39] |
| PEUs | estimated satisfactory biological properties; possible saturation with urethral adhesive proteins | [40,41] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culenova, M.; Bakos, D.; Ziaran, S.; Bodnarova, S.; Varga, I.; Danisovic, L. Bioengineered Scaffolds as Substitutes for Grafts for Urethra Reconstruction. Materials 2019, 12, 3449. https://doi.org/10.3390/ma12203449
Culenova M, Bakos D, Ziaran S, Bodnarova S, Varga I, Danisovic L. Bioengineered Scaffolds as Substitutes for Grafts for Urethra Reconstruction. Materials. 2019; 12(20):3449. https://doi.org/10.3390/ma12203449
Chicago/Turabian StyleCulenova, Martina, Dusan Bakos, Stanislav Ziaran, Simona Bodnarova, Ivan Varga, and Lubos Danisovic. 2019. "Bioengineered Scaffolds as Substitutes for Grafts for Urethra Reconstruction" Materials 12, no. 20: 3449. https://doi.org/10.3390/ma12203449
APA StyleCulenova, M., Bakos, D., Ziaran, S., Bodnarova, S., Varga, I., & Danisovic, L. (2019). Bioengineered Scaffolds as Substitutes for Grafts for Urethra Reconstruction. Materials, 12(20), 3449. https://doi.org/10.3390/ma12203449