Investigations on Aging Behavior and Mechanism of Polyurea Coating in Marine Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2.1. Raw Materials
2.2.2. Properties of Polyurea Coating
2.2. Experimental Method
2.3. Characterization
2.3.1. Contact Angle Measurements
2.3.2. Mechanical Properties
2.3.3. Scanning Electron Microscope
2.3.4. Atomic Force Microscopy
2.3.5. Thermogravimetric Analysis
2.3.6. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy
2.3.7. X-ray Photoelectron Spectroscopic
3. Results
3.1. Surface and Mechanical Properties
3.2. Surface Morphology and Topography
3.3. Thermal Stabilities
3.4. Chemical Changes
4. Conclusions
- 1.
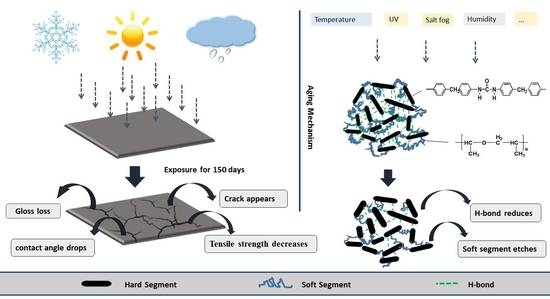
- An experimental investigation was designed and conducted to determine the aging behavior and mechanism of polyurea coating in marine atmosphere. From the appearance and morphology, PCA exhibits macroscopic phenomena such as loss of light and pulverization while the gloss and contact angle decreased by 91.95% and 51.28%, respectively. It was found that the cracks increased with exposure duration, and the area of defects was basically negatively correlated with the contact angle. The surface roughness of PCA increased significantly, the Rrms value reached 49.50 nm, while the Ra was close to 36.3 nm.
- 2.
- FTIR showed that there was a lot of decrease in functional groups such as C–O–C, C=O and C–N bond. The length of the hydrogen bond in the amide-to-amino region remained stable, via calculation, PCB and PCA were both about 2.93 Å. The total hydrogen bonding degree of the urea carbonyl group decreased from 83.41% to 74.56%, indicating that the interaction between the polyurea molecules was weakened.
- 3.
- XPS showed the percentage of C–O–C and C=O content changed greatly, the C–O–C content fell to 24.626%, while the C=O content increased to 15.963%. The soft segment of the PCA was etched more than the hard segment, which increased the degree of microphase separation.
Author Contributions
Funding
Conflicts of Interest
References
- Weibo, H. Spray Polyurea Elastomer Technology; Chemical Industry Press: Beijing, China, 2005. [Google Scholar]
- Iqbal, N.; Tripathi, M.; Parthasarathy, S.; Kumar, D.; Roy, P.K. Polyurea coatings for enhanced blast-mitigation: A review. RSC Adv. 2016, 6, 109706–109717. [Google Scholar] [CrossRef]
- Ma, S.; Van, E.H.; Noordover, B.; Sablong, R.; Van, R.B.; Koning, C. Isocyanate-free approach to water-borne polyurea dispersions and coatings. ChemSusChem 2018, 11, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Grujicic, M.; Pandurangan, B.; He, T.; Cheeseman, B.A.; Yen, C.F.; Randow, C.L. Computational investigation of impact energy absorption capability of polyurea coatings via deformation-induced glass transition. Mater. Sci. Eng. 2010, 527, 7741–7751. [Google Scholar] [CrossRef]
- Gupta, V.; Youssef, G. Orientation-Dependent Impact Behavior of Polymer/EVA Bilayer Specimens at Long Wavelengths. Exp. Mech. 2014, 54, 1133–1137. [Google Scholar] [CrossRef]
- Davies, P.; Evrard, G. Accelerated ageing of polyurethanes for marine applications. Polym. Degrad. Stab. 2007, 92, 1455–1464. [Google Scholar] [CrossRef] [Green Version]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Youssef, G.; Gupta, V. ARTICLES Dynamic tensile strength of polyurea. J. Mater. Res. 2012, 27, 494–499. [Google Scholar] [CrossRef]
- Grujicic, M.; He, T.; Pandurangan, B. Development and parameterization of an equilibrium material model for segmented polyurea. Multidiscip. Model. Mater. Struct. 2011, 7, 96–114. [Google Scholar] [CrossRef]
- Jain, A.; Youssef, G.; Gupta, V. Dynamic tensile strength of polyurea-bonded steel/E-glass composite joints. J. Adhes. Sci. Technol. 2013, 27, 403–412. [Google Scholar] [CrossRef]
- Youssef, G.; Brinson, J.; Whitten, I. The Effect of Ultraviolet Radiation on the Hyperelastic Behavior of Polyurea. J. Polym. Environ. 2017, 26, 183–190. [Google Scholar] [CrossRef]
- Sarva, S.S.; Deschanel, S.; Boyce, M.C.; Chen, W. Stress—Strain behavior of a polyurea and a polyurethane from low to high strain rates. Polymer 2007, 48, 2208–2213. [Google Scholar] [CrossRef]
- Youssef, G.; Whitten, I. Dynamic properties of ultraviolet-exposed polyurea. Mech. Time Depend. Mater. 2016, 21, 351–363. [Google Scholar] [CrossRef]
- Whitten, I.; Youssef, G. The effect of ultraviolet radiation on ultrasonic properties of polyurea. Polym. Degrad. Stab. 2016, 123, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Youssef, G.H. Dynamic Properties of Polyurea. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2011. [Google Scholar]
- Youssef, G.; Gupta, V. Dynamic response of polyurea subjected to nanosecond rise-time stress waves. Mech. Time Depend. Mater. 2012, 16, 317–328. [Google Scholar] [CrossRef]
- Amrollahi, M.; Sadeghi, G.M.M. Assessment of adhesion and surface properties of polyurethane coatings based on non-polar and hydrophobic soft segment. Prog. Org. Coat. 2016, 93, 23–33. [Google Scholar] [CrossRef]
- Malviya, A.K.; Tambe, S.P.; Malviya, A.K.; Tambe, S.P. Accelerated test methods for evaluation of WB coating system comprising of epoxy primer and polyurethane top coat. Prog. Color Color. Coat. 2017, 10, 93–104. [Google Scholar]
- Yang, X.F.; Tallman, D.E.; Bierwagen, G.P.; Croll, S.G.; Rohlik, S. Blistering and degradation of polyurethane coatings under different accelerated weathering tests. Polym. Degrad. Stab. 2002, 77, 103–109. [Google Scholar] [CrossRef]
- Liu, K.; Su, Z.; Miao, S.; Ma, G.; Zhang, S. UV-curable enzymatic antibacterial waterborne polyurethane coating. Biochem. Eng. J. 2016, 113, 107–113. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.; Ji, J.; Yang, Y.; Shen, J.; Ye, M. The hygrothermal aging process and mechanism of the novolac epoxy resin. Compos. Part B 2016, 107, 1–8. [Google Scholar] [CrossRef]
- Aglan, H.; Calhoun, M.; Allie, L. Effect of UV and hygrothermal aging on the mechanical performance of polyurethane elastomers. J. Appl. Polym. Sci. 2008, 108, 558–564. [Google Scholar] [CrossRef]
- Guo, T.X.; Weng, X.Z. Evaluation of the freeze-thaw durability of surface-treated airport pavement concrete under adverse conditions. Constr. Build. Mater. 2019, 206, 519–530. [Google Scholar] [CrossRef]
- Bhargava, S.; Kubota, M.; Lewis, R.D.; Advani, S.G.; Prasad, A.K.; Deitzel, J.M. Ultraviolet, water, and thermal aging studies of a waterborne polyurethane elastomer-based high reflectivity coating. Prog. Org. Coat. 2015, 79, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Boubakri, A.; Guermazi, N.; Elleuch, K.; Ayedi, H.F. Study of UV-aging of thermoplastic polyurethane material. Mater. Sci. Eng. 2010, 527, 1649–1654. [Google Scholar] [CrossRef]
- Liu, P. Artificial Aging Test Analysis of Alkyd and Polyurethane Coating System. Adv. Mater. Res. 2012, 496, 116–120. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiong, J.; Tang, Y.; Zuo, Y. EIS study on failure process of two polyurethane composite coatings. Prog. Org. Coat. 2010, 69, 7–11. [Google Scholar] [CrossRef]
- Rossi, S.; Fedel, M.; Petrolli, S.; Deflorian, F. Accelerated weathering and chemical resistance of polyurethane powder coatings. J. Coat. Technol. Res. 2016, 13, 427–437. [Google Scholar] [CrossRef]
- Yılgör, E.; Yılgör, I.; Yurtsever, E. Hydrogen bonding and polyurethane morphology. I. Quantum mechanical calculations of hydrogen bond energies and vibrational spectroscopy of model compounds. Polymer 2002, 43, 6551–6559. [Google Scholar] [CrossRef]
- Boubakri, A.; Elleuch, K.; Guermazi, N.; Ayedi, H.F. Investigations on hygrothermal aging of thermoplastic polyurethane material. Mater. Des. 2009, 30, 3958–3965. [Google Scholar] [CrossRef]
- Iqbal, N.; Kumar, D.; Roy, P.K. Emergence of time-dependent material properties in chain extended polyureas. J. Appl. Polym. Sci. 2018, 135, 46730. [Google Scholar] [CrossRef]
- Iqbal, N.; Tripathi, M.; Parthasarathy, S.; Kumar, D.; Roy, P.K. Tuning the properties of segmented polyurea by regulating soft-segment length. J. Appl. Polym. Sci. 2018, 135, 46284. [Google Scholar] [CrossRef]
- Iqbal, N.; Tripathi, M.; Parthasarathy, S.; Kumar, D.; Roy, P.K. Aromatic versus Aliphatic: Hydrogen Bonding Pattern in Chain-Extended High-Performance Polyurea. ChemistrySelect 2018, 3, 1976–1982. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Guo, W.; Zou, R. A bilinear constitutive response for polyureas as a function of temperature, strain rate and pressure. J. Appl. Polym. Sci. 2017, 134, 45256. [Google Scholar] [CrossRef]
- Jia, Z.; Amirkhizi, A.V.; Nantasetphong, W.; Nemat-Nasser, S. Experimentally-based relaxation modulus of polyurea and its composites. Mech. Time Depend. Mater. 2016, 20, 155–174. [Google Scholar] [CrossRef] [Green Version]
- Chao, L.; Ma, C.; Xie, Q.; Zhang, G. Self-repairing silicone coating for marine anti-biofouling. J. Mater. Chem. A 2017, 5, 15855–15861. [Google Scholar]
- Zhang, H.; Dun, Y.; Tang, Y.; Zuo, Y.; Zhao, X. Correlation between natural exposure and artificial ageing test for typical marine coating systems. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Y.; Zhang, Z. Synthesis of PPG-TDI-BDO polyurethane and the influence of hard segment content on its structure and antifouling properties. Prog. Org. Coat. 2016, 97, 115–121. [Google Scholar] [CrossRef]
- Meiron, T.S.; Marmur, A.; Saguy, I.S. Contact angle measurement on rough surfaces. J. Colloid Interface Sci. 2004, 274, 637–644. [Google Scholar] [CrossRef]
- Huang, F.; Wei, Q.; Wang, X.; Xu, W. Dynamic contact angles and morphology of PP fibres treated with plasma. Polym. Test. 2006, 27, 22–27. [Google Scholar] [CrossRef]
- Awad, W.H.; Wilkie, C.A. Investigation of the thermal degradation of polyurea: The effect of ammonium polyphosphate and expandable graphite. Polymer 2010, 51, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Ping, L. Studies on the Novel Polyaspartic Ester Based Polyurea Coatings for Marine Concrete Protection. Ph.D. Thesis, Ocean University of China, Shandong, China, 2007. [Google Scholar]
- Lu, P.; Chen, G.H.; Huang, W.B. Degradation of Polyaspartic Polyurea Coating under Different Accelerated Weathering Tests. J. Sichuan Univ. Eng. Sci. Ed. 2007, 39, 92–97. [Google Scholar]
- Lu, P.; Chen, G.; Huang, W. Study on Synthesis, Morphology and Properties of Novel Polyureas Based on Polyaspartic Esters. J. Chem. Eng. Chin. Univ. 2008, 22, 106–112. [Google Scholar]
- Jiang, B.; Tsavalas, J.G.; Sundberg, D.C. Morphology control in surfactant free polyurethane/acrylic hybrid lattices—The special role of hydrogen bonding. Polymer 2018, 139, 107–122. [Google Scholar] [CrossRef]
- Chavan, J.G.; Rath, S.K.; Praveen, S.; Kalletla, S.; Patri, M. Hydrogen bonding and thermomechanical properties of model polydimethylsiloxane based poly(urethane-urea) copolymers: Effect of hard segment content. Prog. Org. Coat. 2016, 90, 350–358. [Google Scholar] [CrossRef]
- Rakshit, P.; Joshi, N.; Jain, R.; Shah, S. Synthesis and Characterization of Polyurea Resin for Dielectric Coating Applications. Polym. Plast. Technol. Eng. 2016, 55, 1683–1692. [Google Scholar] [CrossRef]
- Yang, J.; Wang, G.; Hu, C. Effect of Chain Extenders on Structures and Properties of Aliphatic Polyurethaneureas and Polyureas. Acta Chim. Sin. 2006, 64, 1737–1742. [Google Scholar]
- Chen, T.F.; Siow, K.S.; Ng, P.Y.; Nai, M.H.; Lim, C.T.; Yeop Majlis, B. Ageing properties of polyurethane methacrylate and off-stoichiometry thiol-ene polymers after nitrogen and argon plasma treatment. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]















| Aging Time/d | Wavenumber (cm−1) | Assignment | Area | Percentage of Hydrogen Bonding (%) | ||
|---|---|---|---|---|---|---|
| Xo | Xdiso | Xb | ||||
| 0 | 1629–1644 | Ordered hydrogen bonding | 6.9697 | 57.66 | 83.41 | |
| 1651–1669 | Disordered hydrogen bonding | 3.1131 | 25.75 | |||
| 1681 | free | 2.0055 | ||||
| 150 | 1626–1645 | Ordered hydrogen bonding | 3.1411 | 46.13 | 74.56 | |
| 1651–1666 | Disordered hydrogen bonding | 1.9355 | 28.43 | |||
| 1673–1685 | free | 1.7321 | ||||
| Coating Treatment | Atomic Percentage/% | Atomic Ratio | |||
|---|---|---|---|---|---|
| C | N | O | O/C | N/C | |
| Before the aging | 75.97 | 4.29 | 19.74 | 0.2589 | 0.0565 |
| After aging 150 d | 74.81 | 5.69 | 19.49 | 0.2605 | 0.0761 |
| Element | C–C C–H | C–N | C–O–C | C=O |
|---|---|---|---|---|
| BE(eV) | 284.8 | 285.7 | 286.3 | 288.5 |
| PCB/% | 31.548 | 26.733 | 39.860 | 1.859 |
| PCA/% | 26.648 | 32.763 | 24.626 | 15.963 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, K.; Lyu, P.; Wan, F.; Ma, M. Investigations on Aging Behavior and Mechanism of Polyurea Coating in Marine Atmosphere. Materials 2019, 12, 3636. https://doi.org/10.3390/ma12213636
Che K, Lyu P, Wan F, Ma M. Investigations on Aging Behavior and Mechanism of Polyurea Coating in Marine Atmosphere. Materials. 2019; 12(21):3636. https://doi.org/10.3390/ma12213636
Chicago/Turabian StyleChe, Kaiyuan, Ping Lyu, Fei Wan, and Mingliang Ma. 2019. "Investigations on Aging Behavior and Mechanism of Polyurea Coating in Marine Atmosphere" Materials 12, no. 21: 3636. https://doi.org/10.3390/ma12213636
APA StyleChe, K., Lyu, P., Wan, F., & Ma, M. (2019). Investigations on Aging Behavior and Mechanism of Polyurea Coating in Marine Atmosphere. Materials, 12(21), 3636. https://doi.org/10.3390/ma12213636