Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Biomaterials
2.2. Animals
2.3. Study Design
2.4. Scanning Electronic Microscopy (SEM) Analysis
2.5. Brunauer–Emmett–Teller (BET) Specific Surface Area Analysis
2.6. X-ray Diffraction (XRD) Analysis
2.7. Surgical Procedure
2.8. Euthanasia
3. Histology
3.1. Histomorphometry
- Defect area: defined as the total area of the defect (Region Of Interest, ROI1) Figure 4A
- Regenerated area: defined as the defected area colonized by newly formed bone (ROI2) Figure 4B
- % of regeneration: proportion of ROI2/ROI1.
- % of newly formed bone, % of biomaterial, % of soft tissue within the overall defect area (ROI1)
- The osteoconductivity characterized by the bone to material contact (BMC) defined by the percentage of particles perimeter in contact with newly formed bone within the regenerated area (ROI2).
3.2. Statistical Analysis
4. Results
4.1. Study Design
4.2. SEM Characterization
4.3. Surface Area, Pore Size and Pore Volume Analysis
4.4. XRD Analysis
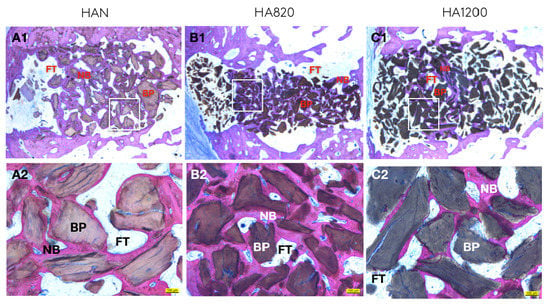
4.5. Descriptive Histology
4.6. Quantitative Histomorphometric Analysis
4.7. Correlation Between Structural Characterization and Biological Performance
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Disclosures
Conflicts of Interest
References
- Esposito, M.; Mg, G.; Rees, J.; Karasoulos, D.; Felice, P.; Alissa, R.; Hv, W. Interventions for replacing missing teeth: Augmentation procedures of the maxillary sinus (Review) SUMMARY OF FINDINGS FOR THE MAIN COMPARISON. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Donos, N.; Mardas, N.; Chadha, V. Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J. Clin. Periodontol. 2008, 35, 173–202. [Google Scholar] [CrossRef] [PubMed]
- Dasmah, A.; Thor, A.; Ekestubbe, A.; Sennerby, L.; Rasmusson, L. Particulate vs. block bone grafts: Three-dimensional changes in graft volume after reconstruction of the atrophic maxilla, a 2-year radiographic follow-up. J. Cranio Maxillofac. Surg. 2012, 40, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Weibull, L.; Widmark, G.; Ivanoff, C.J.; Borg, E.; Rasmusson, L. Morbidity after chin bone harvesting—A retrospective long-term follow-up study. Clin. Implant Dent. Relat. Res. 2009, 11, 149–157. [Google Scholar] [CrossRef]
- Burchardt, H. The Biology of Bone Graft Repair. Clin. Orthop. Related Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Trajkovski, B.; Jaunich, M.; Müller, W.D.; Beuer, F.; Zafiropoulos, G.G.; Houshmand, A. Hydrophilicity, viscoelastic, and physicochemical properties variations in dental bone grafting substitutes. Materials (Basel) 2018, 11, 215. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials - From space holders to innovative biomaterials. J. Cranio Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphatenanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Al-Sanabani, J.S.; Madfa, A.A.; Al-Sanabani, F.A. Application of calcium phosphate materials in dentistry. Int. J. Biomater. 2013, 2013. [Google Scholar] [CrossRef]
- Metsger, D.S.; Dephilip, R.; Hayes, T.G. An Autoradiographic Study of Calcium Phosphate Ceramic Bone Implants in Turkeys. Clin. Orthop. Relat. Res. 1993, 283–294. [Google Scholar] [CrossRef]
- Koepp, H.E.; Schorlemmer, S.; Kessler, S.; Brenner, R.E.; Claes, L.; Günther, K.-P.; Ignatius, A.A. Biocompatibility and osseointegration of β-TCP: Histomorphological and biomechanical studies in a weight-bearing sheep model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70B, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Jun, Y.-K.; Hong, S.-H.; Lee, I.-S.; Kim, H.-E.; Won, Y.Y. Calcium Phosphate Bioceramics with Various Porosities and Dissolution Rates. J. Am. Ceram. Soc. 2004, 85, 3129–3131. [Google Scholar] [CrossRef]
- Chiba, S.; Anada, T.; Suzuki, K.; Saito, K.; Shiwaku, Y.; Miyatake, N.; Baba, K.; Imaizumi, H.; Hosaka, M.; Itoi, E.; et al. Effect of resorption rate and osteoconductivity of biodegradable calcium phosphate materials on the acquisition of natural bone strength in the repaired bone. J. Biomed. Mater. Res. Part A 2016, 104, 2833–2842. [Google Scholar] [CrossRef]
- Ribeiro, N.; Sousa, S.R.; Monteiro, F.J. Influence of crystallite size of nanophased hydroxyapatite on fibronectin and osteonectin adsorption and on MC3T3-E1 osteoblast adhesion and morphology. J. Colloid Interface Sci. 2010, 351, 398–406. [Google Scholar] [CrossRef]
- Gunduz, O.; Erkan, E.M.; Daglilar, S.; Salman, S.; Agathopoulos, S.; Oktar, F.N. Composites of bovine hydroxyapatite (BHA) and ZnO. J. Mater. Sci. 2008, 43, 2536–2540. [Google Scholar] [CrossRef]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes—Comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef]
- Wu, J.; Li, B.; Lin, X. Histological outcomes of sinus augmentation for dental implants with calcium phosphate or deproteinized bovine bone: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 1471–1477. [Google Scholar] [CrossRef]
- Jensen, S.S.; Broggini, N.; Hjørting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and β-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2006, 17, 237–243. [Google Scholar] [CrossRef]
- Accorsi-Mendonça, T.; Conz, M.B.; Barros, T.C.; de Sena, L.Á.; Soares, G.d.; Granjeiro, J.M. Physicochemical characterization of two deproteinized bovine xenografts. Braz. Oral Res. 2008, 22, 5–10. [Google Scholar] [CrossRef]
- Lei, P.; Sun, R.; Wang, L.; Zhou, J.; Wan, L.; Zhou, T.; Hu, Y. A new method for xenogeneic bone graft deproteinization: Comparative study of radius defects in a rabbit model. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Lecloux, G.; Rompen, E. One-Step Approach for Implant Placement and Subantral Bone Regeneration Using Bovine Hydroxyapatite: A 2-to 6-Year Follow-up Study. Int. J. Oral Maxillofac. Implants 2010, 25, 598–606. [Google Scholar] [PubMed]
- Wenz, B.; Oesch, B.; Horst, M. Analysis of the risk of transmitting bovine spongiform encephalopathy through bone grafts derived from bovine bone. Biomaterials 2001, 22, 1599–1606. [Google Scholar] [CrossRef]
- Baldini, N.; de Sanctis, M.; Ferrari, M. Deproteinized bovine bone in periodontal and implant surgery. Dent. Mater. 2011, 27, 61–70. [Google Scholar] [CrossRef]
- Uklejewski, R.; Winiecki, M.; Musielak, G.; Tokłowicz, R. Effectiveness of various deproteinization processes of bovine cancellous bone evaluated via mechano-biostructural properties of produced osteoconductive biomaterials. Biotechnol. Bioprocess Eng. 2015, 20, 259–266. [Google Scholar] [CrossRef]
- Goller, G.; Oktar, F.N.; Agathopoulos, S.; Tulyaganov, D.U.; Ferreira, J.M.F.; Kayali, E.S.; Peker, I. Effect of sintering temperature on mechanical and microstructural properties of bovine hydroxyapatite (BHA). J. Sol-Gel Sci. Technol. 2006, 37, 111–115. [Google Scholar] [CrossRef]
- Witek, L.; Smay, J.; Silva, N.R.F.A.; Guda, T.; Ong, J.L.; Coelho, P.G. Sintering effects on chemical and physical properties of bioactive ceramics. J. Adv. Ceram. 2013, 2, 274–284. [Google Scholar] [CrossRef]
- Knowles, J.C.; Horton, J.A.; Bonfield, W. Rietveld Analysis on the Effect of Sintering Conditions on the Structure of Hydroxyapatite. Bioceramics 1994, 23–28. [Google Scholar] [CrossRef]
- Barone, A.; Todisco, M.; Ludovichetti, M.; Gualini, F.; Aggstaller, H.; Torres-Lagares, D.; Rohrer, M.; Prasad, H.; Kenealy, J. A Prospective, Randomized, Controlled, Multicenter Evaluation of Extraction Socket Preservation Comparing Two Bovine Xenografts: Clinical and Histologic Outcomes. Int. J. Periodontics Restor. Dent. 2013, 33, 795–802. [Google Scholar] [CrossRef]
- Spies, C.K.G.; Schnürer, S.; Gotterbarm, T.; Breusch, S.J. Efficacy of Bone SourceTM and CementekTM in comparison with EndobonTM in critical size metaphyseal defects, using a minipig model. J. Appl. Biomater. Biomech. 2010, 8, 175–185. [Google Scholar] [CrossRef]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radiographic Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 1089–1096. [Google Scholar] [CrossRef]
- Takauti, C.A.Y.; Futema, F.; Junior, R.B.d.; Abrahao, A.C.; Costa, C.; Queiroz, C.S. Assessment of Bone Healing in Rabbit Calvaria grafted with three different Biomaterials. Braz. Dent. J. 2014, 25, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Joschek, S.; Nies, B.; Krotz, R.; Göpferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Figueiredo, M.; Fernando, A.; Martins, G.; Freitas, J.; Judas, F.; Figueiredo, H. Effect of the calcination temperature on the composition and microstructure of hydroxyapatite derived from human and animal bone. Ceram. Int. 2010, 36, 2383–2393. [Google Scholar] [CrossRef]
- Etok, S.E.; Valsami-Jones, E.; Wess, T.J.; Hiller, J.C.; Maxwell, C.A.; Rogers, K.D.; Manning, D.A.C.; White, M.L.; Lopez-Capel, E.; Collins, M.J.; et al. Structural and chemical changes of thermally treated bone apatite. J. Mater. Sci. 2007, 42, 9807–9816. [Google Scholar] [CrossRef]
- Li, X.; van Blitterswijk, C.A.; Feng, Q.; Cui, F.; Watari, F. The effect of calcium phosphate microstructure on bone-related cells in vitro. Biomaterials 2008, 29, 3306–3316. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Kruyt, M.C.; Juhl, M.V.; Clyens, S.; Martinetti, R.; Dolcini, L.; Theilgaard, N.; van Blitterswijk, C.A. Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J. Orthop. Res. 2008, 26, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Rouahi, M.; Champion, E.; Gallet, O.; Jada, A.; Anselme, K. Physico-chemical characteristics and protein adsorption potential of hydroxyapatite particles: Influence on in vitro biocompatibility of ceramics after sintering. Colloids Surfaces B Biointerfaces 2006, 47, 10–19. [Google Scholar] [CrossRef]
- Espanol, M.; Mestres, G.; Luxbacher, T.; Dory, J.B.; Ginebra, M.P. Impact of Porosity and Electrolyte Composition on the Surface Charge of Hydroxyapatite Biomaterials. ACS Appl. Mater. Interfaces 2016, 8, 908–917. [Google Scholar] [CrossRef] [Green Version]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W. Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo. J. Biomed. Mater. Res. Part A 2008, 85, 777–786. [Google Scholar] [CrossRef]
- Lambert, F.; Bacevic, M.; Layrolle, P.; Schüpbach, P.; Drion, P.; Rompen, E.; Schupbach, P.; Drion, P.; Rompen, E. Impact of biomaterial microtopography on bone regeneration: Comparison of three hydroxyapatites. Clin. Oral Implants Res. 2017, 28, e201–e207. [Google Scholar] [CrossRef]
- Kurkcu, M.; Benlidayi, M.E.; Cam, B.; Sertdemir, Y. Anorganic Bovine-Derived Hydroxyapatite vs β-Tricalcium Phosphate in Sinus Augmentation: A Comparative Histomorphometric Study. J. Oral Implantol. 2012, 38, 519–526. [Google Scholar] [CrossRef]
- Rompen, E.; Lambert, F.; Lecloux, G.; Moniotte, P. Materiau De Regeneration Osseuse Et Son Procede De Fabrication. Pat. WO 2015/049336 A1, 2015. [Google Scholar]
- Wang, S.; Liu, Y.; Fang, D.; Shi, S. The miniature pig: A useful large animal model for dental and orofacial research. Oral Dis. 2007, 13, 530–537. [Google Scholar] [CrossRef]
- Cheary, B.Y.R.W.; Coelho, A. A Fundamental Parameters Approach to X-ray Line-Profile Fitting. J. Appl. Crystallogr. 1992, 109–121. [Google Scholar] [CrossRef]
- Krill, C.E.; Haberkorn, R.; Birringer, R. Specification of microstructure and characterization by scattering techniques, Handb. Nanostruct. Mater. Nanotechnol. 2000, 155–211. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues*. J. Oral Pathol. Med. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Crystallogr. Sect. B 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Amara, M.B.; Vlasse, M.; le Flem, G.; Hagenmuller, P. Structure of the low-temperature variety of calcium sodium orthophosphate, NaCaPO4. Acta Crystallogr. Sect. C 1983, 39, 1483–1485. [Google Scholar] [CrossRef]
- Shen, C.H.; Liu, R.S.; Lin, J.G.; Huang, C.Y. Phase stability study of La1.2Ca1.8Mn2O7. Mater. Res. Bull. 2001, 36, 1139–1148. [Google Scholar] [CrossRef]
- Yetmez, M.; Erkmen, Z.E.; Kalkandelen, C.; Ficai, A.; Oktar, F.N. Sintering effects of mullite-doping on mechanical properties of bovine hydroxyapatite. Mater. Sci. Eng. C 2017, 77, 470–475. [Google Scholar] [CrossRef]
- Kusrini, E.; Sontang, M. Characterization of x-ray diffraction and electron spin resonance: Effects of sintering time and temperature on bovine hydroxyapatite. Radiat. Phys. Chem. 2012, 81, 118–125. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31. [Google Scholar] [CrossRef]
- Riachi, F.; Naaman, N.; Tabarani, C.; Aboelsaad, N.; Aboushelib, M.N.; Berberi, A.; Salameh, Z. Influence of material properties on rate of resorption of two bone graft materials after sinus lift using radiographic assessment. Int. J. Dent. 2012, 2012. [Google Scholar] [CrossRef]
- Fernández, M.P.R.; Gehrke, S.A.; Martinez, C.P.A.; Guirado, J.L.C.; de Aza, P.N. SEM-EDX study of the degradation process of two xenograft materials used in sinus lift procedures. Materials (Basel) 2017, 10, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Patel, N.; Gibson, I.R.; Ke, S.; Best, S.M.; Bonfield, W.; Materials, B.; Mary, Q.; College, W.; Road, M.E. Calcining infuence on the powder properties of hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 2, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Y.; Ramesh, S.; Aw, K.L.; Yeo, W.H.; Hamdi, M.; Sopyan, I. Effect of powder calcination on the sintering of hydroxyapatite. Med. J. Malaysia 2008, 63 (Suppl A), 87–88. [Google Scholar] [CrossRef]
- Wang, A.J.; Lu, Y.P.; Zhu, R.F.; Li, S.T.; Xiao, G.Y.; Zhao, G.F.; Xu, W.H. Effect of sintering on porosity, phase, and surface morphology of spray dried hydroxyapatite microspheres. J. Biomed. Mater. Res. Part A 2008, 87, 557–562. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2000, 22, 87–96. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Gortemaker, A.; van Hof, M.A. Critical reviews in oral biology & medicine Implant Surface Roughness and Bone Healing. J. Dent. Res. 2006, 496–501. [Google Scholar] [CrossRef]
- Molly, L.; Vandromme, H.; Quirynen, M.; Schepers, E.; Adams, J.L.; van Steenberghe, D. Bone Formation Following Implantation of Bone Biomaterials Into Extraction Sites. J. Periodontol. 2008, 79, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Leonard, A.; Lecloux, G.; Sourice, S.; Pilet, P.; Rompen, E. A Comparison of Three Calcium Phosphate–Based Space Fillers in Sinus Elevation: A Study in Rabbits. Int. J. Oral Maxillofac. Implants 2013, 28, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| HAN | HA820 | HA1200 | |
|---|---|---|---|
| Surface area (m2/g) | ~36 | ~4.27 | ~0.27 |
| Pore size (nm) | ~10 | ~5.2 | ~4.5 |
| Total pore volume (cm3/g) | ~0.13 | ~0.007 | ~0.003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Carvalho, B.; Rompen, E.; Lecloux, G.; Schupbach, P.; Dory, E.; Art, J.-F.; Lambert, F. Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite. Materials 2019, 12, 3946. https://doi.org/10.3390/ma12233946
De Carvalho B, Rompen E, Lecloux G, Schupbach P, Dory E, Art J-F, Lambert F. Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite. Materials. 2019; 12(23):3946. https://doi.org/10.3390/ma12233946
Chicago/Turabian StyleDe Carvalho, Bruno, Eric Rompen, Geoffrey Lecloux, Peter Schupbach, Emilie Dory, Jean-François Art, and France Lambert. 2019. "Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite" Materials 12, no. 23: 3946. https://doi.org/10.3390/ma12233946
APA StyleDe Carvalho, B., Rompen, E., Lecloux, G., Schupbach, P., Dory, E., Art, J. -F., & Lambert, F. (2019). Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite. Materials, 12(23), 3946. https://doi.org/10.3390/ma12233946