Optical Properties of Oxidized Plasma-Polymerized Organosilicones and Their Correlation with Mechanical and Chemical Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasma Polymerization Technique
2.2. Thin Film Characterization
2.3. Ellipsometric Spectra Analysis
3. Results
3.1. Chemical Analysis of Thin Films
3.2. Deposition Rate
3.3. Surface Topography of Thin Films
3.4. Optical Properties of Thin Films
3.5. Correlation between Optical and Mechanical Properties of Thin Films
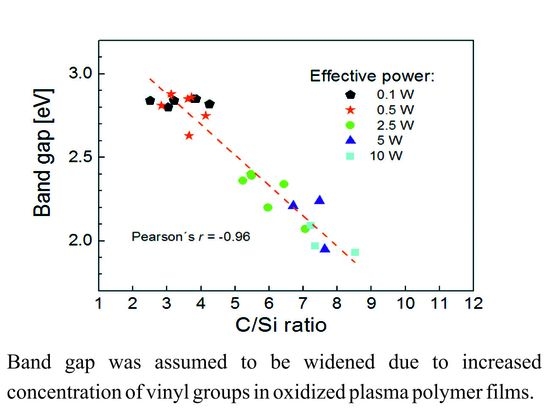
3.6. Correlation between Optical and Chemical Properties of Thin Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Optical Coatings Industry. PRNewswire 2018. Available online: https://www.marketwatch.com/press-release/global-optical-coatings-industry-2018-06-25 (accessed on 15 January 2019).
- Greene, J.E. Review Article: Tracing the recorded history of thin-film sputter deposition: From the 1800s to 2017. J. Vac. Sci. Technol. A 2017, 35, 05C204. [Google Scholar] [CrossRef]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. A 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of plasma polymerization–reviewed from a chemical point of view. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mat. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Bussey, D.; Perina, V.; Jones, F.R.; Cech, V. Effect of chemical modification on the mechanical properties of plasma polymerized organosilicones. Prog. Org. Coat. 2018, 119, 85–90. [Google Scholar] [CrossRef]
- Cech, V.; Zemek, J.; Perina, V. Chemistry of plasma-polymerized vinyltriethoxysilane controlled by deposition conditions. Plasma Proces. Polym. 2008, 5, 745–752. [Google Scholar] [CrossRef]
- Palesch, E.; Knob, A.; Plichta, T.; Cech, V. Functional interlayers with controlled adhesion developed for polymer composites. Thin Solid Films 2018, 656, 37–43. [Google Scholar] [CrossRef]
- Cech, V.; Lichovnikova, S.; Sova, J.; Studynka, J. Surface free energy of silicon-based plasma polymer films. In Silanes and Other Coupling Agents; Mittal, K.L., Ed.; VSP: Leiden, The Netherlands, 2009; Volume 5, pp. 333–348. [Google Scholar]
- Cech, V.; Lukes, J.; Palesch, E.; Lasota, T. Elastic modulus and hardness of plasma-polymerized organosilicones evaluated by nanoindentation techniques. Plasma Process. Polym. 2015, 12, 864–881. [Google Scholar] [CrossRef]
- Cech, V. Plasma polymer film as a model interlayer for polymer composites. IEEE Trans. Plasma Sci. 2006, 34, 1148–1155. [Google Scholar] [CrossRef]
- Yasuda, H. Plasma Polymerization; Academic Press: Orlando, FL, USA, 1985; pp. 72–77. [Google Scholar]
- Studynka, J.; Cech, V. Aging of silicon-based dielectric coatings deposited by plasma polymerization. Thin Solid Films 2011, 519, 2168–2171. [Google Scholar] [CrossRef]
- Jellison, G.E., Jr.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Stat. Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Jellison, G.E., Jr. The calculation of thin film parameters from spectroscopic ellipsometry data. Thin Solid Films 1996, 290–291, 40–45. [Google Scholar] [CrossRef]
- Cech, V.; Studynka, J.; Conte, N.; Perina, V. Physico-chemical properties of plasma-polymerized tetravinylsilane. Surf. Coat. Technol. 2007, 201, 5512–5517. [Google Scholar] [CrossRef]
- Cech, V.; Studynka, J.; Janos, F.; Perina, V. Influence of oxygen on the chemical structure of plasma polymer films deposited from a mixture of tetravinylsilane and oxygen gas. Plasma Process. Polym. 2007, 4, S776–S780. [Google Scholar] [CrossRef]
- Inagaki, N. Plasma Surface Modification and Plasma Polymerization; Technomic Publ.: Lancaster, Pennsylvania, 1996; pp. 127–134. [Google Scholar]
- Talebian, E.; Talebian, M. A general review on the derivation of Clausius–Mossotti relation. Optik 2013, 124, 2324–2326. [Google Scholar] [CrossRef]
- Mitchell, B.S. Materials Engineering and Science for Chemical and Materials Engineers; Wiley: Hoboken, NJ, USA, 2004; p. 851. [Google Scholar]
- Phillips, J.C. Chemical models of energy bands. In Handbook of Semiconductors; Moss, T.S., Ed.; Elsevier: Amsterdam, The Netherlands, 1992; pp. 47–57. [Google Scholar]
- Shao, J. Mathematical Statistics; Springer-Verlag: New York, NY, USA, 2004. [Google Scholar]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Publishing: Pacific Grove, CA, USA, 1996. [Google Scholar]
- Kulisch, W. Deposition of Diamond-Like Superhard Materials; Springer: Berlin, Germany, 1999; pp. 27–35. [Google Scholar]
- Casiraghi, C.; Robertson, J.; Ferrari, A.C. Diamond-like carbon for data and beer storage. Mater. Today 2007, 10, 44–53. [Google Scholar]
- Casiraghia, C.; Piazzaa, F.; Ferraria, A.C.; Gramboleb, D.; Robertson, J. Bonding in hydrogenated diamond-like carbon by Raman spectroscopy. Diamond Relat. Mater. 2005, 14, 1098–1102. [Google Scholar]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cechalova, B.; Branecky, M.; Klapetek, P.; Cech, V. Optical Properties of Oxidized Plasma-Polymerized Organosilicones and Their Correlation with Mechanical and Chemical Parameters. Materials 2019, 12, 539. https://doi.org/10.3390/ma12030539
Cechalova B, Branecky M, Klapetek P, Cech V. Optical Properties of Oxidized Plasma-Polymerized Organosilicones and Their Correlation with Mechanical and Chemical Parameters. Materials. 2019; 12(3):539. https://doi.org/10.3390/ma12030539
Chicago/Turabian StyleCechalova, Bozena, Martin Branecky, Petr Klapetek, and Vladimir Cech. 2019. "Optical Properties of Oxidized Plasma-Polymerized Organosilicones and Their Correlation with Mechanical and Chemical Parameters" Materials 12, no. 3: 539. https://doi.org/10.3390/ma12030539
APA StyleCechalova, B., Branecky, M., Klapetek, P., & Cech, V. (2019). Optical Properties of Oxidized Plasma-Polymerized Organosilicones and Their Correlation with Mechanical and Chemical Parameters. Materials, 12(3), 539. https://doi.org/10.3390/ma12030539