Understanding Free Volume Characteristics of Ethylene-Propylene-Diene Monomer (EPDM) through Molecular Dynamics Simulations
Abstract
:1. Introduction
2. MD Simulation Methods
2.1. Molecular Dynamics Simulations
2.1.1. Potentials
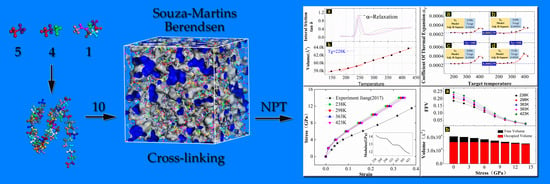
2.1.2. Creating a Cross-Linked MD Model
2.2. Verification of the MD Model
2.2.1. Glass Transition Temperature
2.2.2. Gas Diffusivity
2.2.3. Mechanical Property of EPDM
2.3. Molecular Simulation of Free Volume
3. Results and Discussion
3.1. Principles of Superposition between Time, Temperature and Strain
3.2. The Dependence of EPDM’s Free Volume on Temperature
3.3. The Dependence of EPDM’s Free Volume on Load Magnitude
3.4. The Feasibility of the Superposition between Temperature and Strain
4. Conclusions
- The MD simulation results for the glass transition temperature and stress–strain curve were compared with values in the literature, and there is good agreement. The simulated O2 diffusion coefficient is closer to the experimental value than the Rutherford’s simulation, as the cross-linked EPDM model in the current study is closer to the experimental one.
- The fractional free volume is proportional to temperature only when the target temperature is lower than 330 K; the temperature expansion coefficient is approximately 0.000239, and while higher than 330 K, the temperature expansion coefficient is no longer constant, and this is consistent with the applicable temperature range of WLF equation, ~ + 100.
- The fractional free volume of EPDM decreases exponentially with increasing strain magnitude, which can be expressed as .
- The free volume predicted by temperature–strain superposition has a significant error compared with the MD simulation results. The error is acceptable only when the reference temperature is 298 K and the target temperature is 333 K, so the superposition of temperature and strain magnitude is feasible only in a particular situation.
- TTSP is applicable for EPDM at temperatures below 330 K; the assumptions of TSSP have proven to be unsuitable for EPDM. The contribution of temperature and strain to free volume can barely be superimposed at the appropriate reference temperature, so the applicability of TTSSP to EPDM is also limited.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paroli, R.M.; Dutt, O.; Delgado, A.H.; Mech, M.N. Characterization of ethylene-propylene-diene monomer (epdm) roofing membranes using thermogravimetry and dynamic mechanical analysis. Thermochim. Acta 1991, 182, 303–317. [Google Scholar] [CrossRef]
- Schurch, M. Small but important–gaskets for tunnel segments. In Proceedings of the International Symposiumon on Underground Excavation and Tunnelling 2006.
- Cui, T.; Chao, Y.J.; Van Zee, J.W. Stress relaxation behavior of EPDM seals in polymer electrolyte membrane fuel cell environment. Int. J. Hydrog. Energy 2012, 37, 13478–13483. [Google Scholar] [CrossRef]
- Natali, M.; Rallini, M.; Puglia, D.; Kenny, J.; Torre, L. EPDM based heat shielding materials for Solid Rocket Motors: A comparative study of different fibrous reinforcements. Polym. Degrad. Stabil. 2013, 98, 2131–2139. [Google Scholar] [CrossRef]
- Wu, H.-N.; Huang, R.-Q.; Sun, W.-J.; Shen, S.-L.; Xu, Y.-S.; Liu, Y.-B.; Du, S.-J. Leaking behavior of shield tunnels under the Huangpu River of Shanghai with induced hazards. Nat. Hazards 2014, 70, 1115–1132. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H. Analysis of Leakage Paths Induced by Longitudinal Differential Settlement of the Shield-driven Tunneling. DEStech Transactions on Engineering and Technology Research. In Proceedings of the The 3rd International Conference on Engineering, Technology, and Industrial Application, Surakarta, Indonesia, 7–8 December 2016. [Google Scholar] [CrossRef]
- Wu, Z. Study on Some Key Problem on the Durability of Shield Tunnel under River; TongJi University: Shanghai, China, 2008. [Google Scholar]
- Xiaochun, Z.; Jianshe, Q.; Wei, Z.; Fanlu, M. Durability tests and analysis of the waterproof material for joint seam of shield tunnel. Chin. J. Undergr. Space Eng. 2011, 7, 281–285. [Google Scholar]
- Shi, C.; Cao, C.; Lei, M.; Peng, L.; Shen, J. Time-dependent performance and constitutive model of EPDM rubber gasket used for tunnel segment joints. Tunn. Undergr. Space Technol. 2015, 50, 490–498. [Google Scholar] [CrossRef]
- Van Gurp, M.; Palmen, J. Time-temperature superposition for polymeric blends. Rheol. Bull. 1998, 67, 5–8. [Google Scholar]
- Luo, W.B.; Yang, T.Q.; An, Q.L. Time-temperature-stress equivalence and its application to nonlinear viscoelastic materials. Acta Mech. Solida Sin. 2001, 14, 195–199. [Google Scholar]
- Ginic-Markovic, M.; Dutta, N.K.; Dimopoulos, M.; Roy Choudhury, N.; Matisons, J.G. Viscoelastic behaviour of filled, and unfilled, EPDM elastomer. Thermochim. Acta 2000, 357–358, 211–216. [Google Scholar] [CrossRef]
- Ronan, S.; Alshuth, T.; Jerrams, S.; Murphy, N. Long-term stress relaxation prediction for elastomers using the time–temperature superposition method. Mater. Des. 2007, 28, 1513–1523. [Google Scholar] [CrossRef]
- Wang, B.; Fancey, K.S. Application of time-stress superposition to viscoelastic behavior of polyamide 6,6 fiber and its “true” elastic modulus. J. Appl. Polym. Sci. 2017, 134, 9. [Google Scholar] [CrossRef]
- Irfan, M.S.; Gill, Y.Q.; Hashmi, M.; Ullah, S.; Saeed, F.; Qaiser, A.A. Long-term stress relaxation behavior of Polyaniline-EPDM blends using the time-temperature-strain superposition method. Mater. Res. Express 2019, 6, 8. [Google Scholar] [CrossRef]
- Wu, C.F.; Xu, W.J. Atomistic molecular modelling of crosslinked epoxy resin. Polymer 2006, 47, 6004–6009. [Google Scholar] [CrossRef]
- Wu, C.F.; Xu, W.J. Atomistic simulation study of absorbed water influence on structure and properties of crosslinked epoxy resin. Polymer 2007, 48, 5440–5448. [Google Scholar] [CrossRef]
- Rutherford, S.W.; Limmer, D.T.; Smith, M.G.; Honnell, K.G. Gas transport in ethylene–propylene–diene (EPDM) elastomer: Molecular simulation and experimental study. Polymer 2007, 48, 6719–6727. [Google Scholar] [CrossRef]
- Varshney, V.; Patnaik, S.S.; Roy, A.K.; Farmer, B.L. A molecular dynamics study of epoxy-based networks: Cross-linking procedure and prediction of molecular and material properties. Macromolecules 2008, 41, 6837–6842. [Google Scholar] [CrossRef]
- Maly, M.; Posocco, P.; Fermeglia, M.; Pricl, S. Scripting approach in hybrid organic–inorganic condensation simulation: The GPTMS proof-of-concept. Mol. Simul. 2008, 34, 1215–1236. [Google Scholar] [CrossRef]
- Aghadavoudi, F.; Golestanian, H.; Beni, Y.T. Investigating the Effects of Resin Crosslinking Ratio on Mechanical Properties of Epoxy-Based Nanocomposites Using Molecular Dynamics. Polym. Compos. 2017, 38, E433–E442. [Google Scholar] [CrossRef]
- Rabe, J.P.; Buchholz, S. Direct observation of molecular-structure and dynamics at the interface between a solid wall and an organic solution by scanning tunneling microscopy. Phys. Rev. Lett. 1991, 66, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Mackay, M.E.; Dao, T.T.; Tuteja, A.; Ho, D.L.; Van Horn, B.; Kim, H.C.; Hawker, C.J. Nanoscale effects leading to non-Einstein-like decrease in viscosity. Nat. Mater. 2003, 2, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Brostow, W. Time-stress correspondence in viscoelastic materials: An equation for the stress and temperature shift factor. Mater. Res. Innov. 2000, 3, 347–351. [Google Scholar] [CrossRef]
- Golzar, K.; Amjad-Iranagh, S.; Amani, M.; Modarress, H. Molecular simulation study of penetrant gas transport properties into the pure and nanosized silica particles filled polysulfone membranes. J. Membr. Sci. 2014, 451, 117–134. [Google Scholar] [CrossRef]
- Peng, Z.W.; Ewig, C.S.; Hwang, M.J.; Waldman, M.; Hagler, A.T. Derivation of class II force fields. 4. van der Waals parameters of alkali metal cations and halide anions. J. Phys. Chem. A 1997, 101, 7243–7252. [Google Scholar] [CrossRef]
- van Duin, M.; Orza, R.; Peters, R.; Chechik, V. Mechanism of Peroxide Cross-Linking of EPDM Rubber. In Polymer Networks: Synthesis, Properties, Theory and Applications; Patrickios, C.S., Ed.; Wiley-V C H Verlag Gmbh: Weinheim, Germany, 2010; Volume 291–292, p. 66. [Google Scholar]
- Zachary, M.; Camara, S.; Whitwood, A.C.; Gilbert, B.C.; van Duin, M.; Meier, R.J.; Chechik, V. EPR study of persistent free radicals in cross-linked EPDM rubbers. Eur. Polym. J. 2008, 44, 2099–2107. [Google Scholar] [CrossRef]
- Seo, A.; Kurimoto, H. Ethylene Propylene Diene Monomer Composition Used for Manufacturing e.g., Gasket, Contains Ethylene Propylene Diene Polymer Having Specific Mooney Viscosity, Carbon Black, Process Oil, and Peroxide Vulcanizing Agent. WO Patent WO2017199581-A1, 23 November 2017. [Google Scholar]
- Torchia, S.R.; Hudson, R.; Romanova, T. Stabilized Polymer Composition Comprises Polymer Selected from Polyolefin, Styrenic Polymer, and/or Poly(meth)acrylate, and Stabilizer Composition Comprising Antacid, Organic Acid-Metal Salt, and Primary Antioxidant. U.S. Patent US2018312665-A1, 1 November 2018. [Google Scholar]
- Gu, Z.; Zhang, X.; Bao, C.; Xue, M.; Wang, H.; Tian, X.Y. Crosslinking-Dependent Relaxation Dynamics in Ethylene-Propylene-Diene (EPDM) Terpolymer above the Glass Transition Temperature. J. Macromol. Sci. Part B-Phys. 2015, 54, 618–627. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.; Wang, W.; Zhu, S.; Guo, L.; Zhang, Z.; Li, P. Effects of different functional groups in graphene nanofiber on the mechanical property of polyvinyl alcohol composites by the molecular dynamic simulations. J. Mol. Liq. 2019, 277, 261–268. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, X.; Xu, J.-S.; Zhang, Z.-S.; Du, H.-Y. Quasi-static compression mechanical properties of EPDM material after pyrolysis. J. Aerosp. Power 2017, 32, 114–119. [Google Scholar]
- Lee, S.; Mattice, W.L. A “phantom bubble” model for the distribution of free volume in polymers. Comput. Theor. Polym. Sci. 1999, 9, 57–61. [Google Scholar] [CrossRef]
- Madkour, T.M. Development of the molecular design rules of ultra-permeable poly 1-(trimethylsilyl)-1-propyne membranes. Polymer 2000, 41, 7489–7497. [Google Scholar] [CrossRef]














| Reference Strain | Reference Fractional Free Volume (f0) | A | A | B | B | Statistics | Statistics |
|---|---|---|---|---|---|---|---|
| Value | Standard Error | Value | Standard Error | Reduced Chi-Sqr | Adj. R-Square | ||
| ε0 = 0 | 0.203866 | 0.2048 | 0.00311 | −0.20862 | 0.00365 | 2.14 × 10−6 | 0.99944 |
| ε0 = 0.01 | 0.187926 | 0.19096 | 0.0037 | −0.19027 | 0.00375 | 3.04 × 10−6 | 0.99937 |
| ε0 = 0.04 | 0.153478 | 0.15704 | 0.00422 | −0.15496 | 0.00375 | 3.04 × 10−6 | 0.99937 |
| ε0 = 0.09 | 0.114238 | 0.11673 | 0.00453 | −0.11623 | 0.00375 | 3.04 × 10−6 | 0.99937 |
| ε0 = 0.15 | 0.080902 | 0.08151 | 0.00437 | −0.08356 | 0.00375 | 3.04 × 10−6 | 0.99937 |
| ε0 = 0.21 | 0.052951 | 0.05548 | 0.00386 | −0.05637 | 0.00375 | 3.04 × 10−6 | 0.99937 |
| ε0 = 0.27 | 0.03418 | 0.03769 | 0.00323 | −0.03622 | 0.00375 | 3.04 × 10−6 | 0.99937 |
| ε0 = 0.33 | 0.024388 | 0.02608 | 0.00264 | −0.02616 | 0.00375 | 3.04 × 10−6 | 0.99937 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, Y.; Tao, M. Understanding Free Volume Characteristics of Ethylene-Propylene-Diene Monomer (EPDM) through Molecular Dynamics Simulations. Materials 2019, 12, 612. https://doi.org/10.3390/ma12040612
Wang Y, Yang Y, Tao M. Understanding Free Volume Characteristics of Ethylene-Propylene-Diene Monomer (EPDM) through Molecular Dynamics Simulations. Materials. 2019; 12(4):612. https://doi.org/10.3390/ma12040612
Chicago/Turabian StyleWang, Yajian, Yuyou Yang, and Mingjiang Tao. 2019. "Understanding Free Volume Characteristics of Ethylene-Propylene-Diene Monomer (EPDM) through Molecular Dynamics Simulations" Materials 12, no. 4: 612. https://doi.org/10.3390/ma12040612
APA StyleWang, Y., Yang, Y., & Tao, M. (2019). Understanding Free Volume Characteristics of Ethylene-Propylene-Diene Monomer (EPDM) through Molecular Dynamics Simulations. Materials, 12(4), 612. https://doi.org/10.3390/ma12040612