Recent Advances of Hierarchical and Sequential Growth of Macromolecular Organic Structures on Surface
Abstract
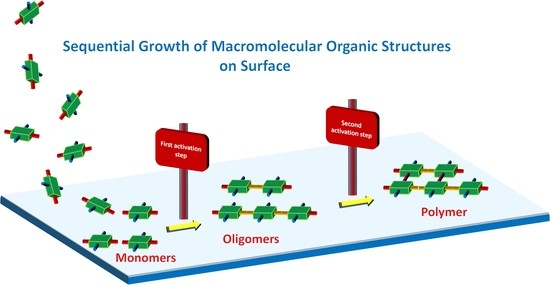
:1. Introduction
2. Coupling Modes Used for the Design of 2D Covalent Networks
2.1. Hierarchical Growth of Macromolecular Architectures Based on Successive Ullmann Couplings
2.2. Sequential Growth Based on the Boroxine Formation/Ullmann Coupling Combination
2.3. Sequential Growth Based on the Boroxine Formation/Imine Combination
2.4. Hierarchical Growth Based on the Coordination Polymer/Phthalocyanine Formation
3. Coupling Modes Used for the Design of 1D Macromolecular Organic Structures
3.1. Sequential Growth Based on the Ullmann Coupling/Aromatization Combination
3.2. Sequential Growth Based on the Coordination Polymer/Glaser Coupling Combination
3.3. Sequential Growth Based on the Glaser Coupling/Dehydrogenative Coupling Combination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, F.; Liu, H.; Mathe, S.D.R.; Dong, A.; Zhang, J. Covalent organic frameworks: From materials design to biomedical application. Nanomaterials 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.S.; Bein, T. Covalent organic frameworks: Structures, synthesis, and applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Lackinger, M.; Griessl, S.; Markert, T.; Jamitzky, F.; Heckl, W.M. Self-assembly of benzene-dicarboxylic acid isomers at the liquid solid interface: Steric aspects of hydrogen bonding. J. Phys. Chem. B 2004, 108, 13652–13655. [Google Scholar] [CrossRef]
- Griessl, S.; Lackinger, M.; Edelwirth, M.; Hietschold, M.; Heckl, W.M. Self-assembled two-dimensional molecular host-guest architectures from trimesic acid. Single Mol. 2002, 3, 25–31. [Google Scholar] [CrossRef]
- Kühne, D.; Klappenberger, F.; Decker, R.; Schlickum, U.; Brune, H.; Klyatskaya, D.; Ruben, M.; Barth, J.V. High-quality 2D metal-organic coordination network providing giant cavities within mesoscale domains. J. Am. Chem. Soc. 2009, 131, 3881–3883. [Google Scholar] [CrossRef] [PubMed]
- Langner, A.; Tait, S.L.; Lin, N.; Rajadurai, C.; Ruben, M.; Kern, K. Self-recognition and self-selection in multicomponent supramolecular coordination networks on surfaces. Proc. Natl. Acad. Sci. USA 2007, 104, 17927–17930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, J.V. Molecular architectonic on metal surfaces. Annu. Rev. Phys. Chem. 2007, 58, 375–407. [Google Scholar] [CrossRef]
- De Feyter, S.; De Schryver, F.C. Two-dimensional supramolecular self-assembly probed by scanning tunneling microscopy. Chem. Soc. Rev. 2003, 32, 139–150. [Google Scholar] [CrossRef] [Green Version]
- Barth, J.V. Fresh perspectives for surface coordination chemistry. Surf. Sci. 2009, 603, 1533–1541. [Google Scholar] [CrossRef]
- Koudia, M.; Nardi, E.; Siri, O.; Abel, M. On-surface synthesis of covalent coordination polymers on micrometer scale. Nano Res. 2017, 10, 933–940. [Google Scholar] [CrossRef]
- Nakanishi, T. Supramolecular soft and hard materials based on self-assembly algorithms of alkyl-conjugated fullerenes. Chem. Commun. 2010, 46, 3425–3436. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Pfeffermann, M.; Liang, H.; Zheng, Z.; Zhu, X.; Zhang, J.; Feng, X. Large-area, free-standing, two-dimensional supramolecular polymer single-layer sheets for highly efficient electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 12058–12063. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Mendecki, L.; Mirica, K.A. Conductive two-dimensional metal–organic frameworks as multifunctional materials. Chem. Commun. 2018, 54, 7873–7891. [Google Scholar] [CrossRef] [PubMed]
- Gourdon, A. On-surface covalent coupling in ultrahigh vacuum. Angew. Chem. Int. Ed. 2008, 47, 6950–6953. [Google Scholar] [CrossRef] [PubMed]
- Perepichka, D.F.; Rosei, F. Extending polymer conjugation into the second dimension. Science 2009, 323, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Lopez, M.F.; Martin-Gago, J.A. On-surface synthesis of cyclic organic molecules. Chem. Soc. Rev. 2011, 40, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Franc, G.; Gourdon, A. Covalent networks through on-surface chemistry in ultrahigh vacuum: State-of-the-art and recent developments. Phys. Chem. Chem. Phys. 2011, 13, 14283–14292. [Google Scholar] [CrossRef] [PubMed]
- Grill, L.; Dyer, M.; Lafferentz, L.; Persson, M.; Peters, M.V.; Hecht, S. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2007, 2, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Bieri, M.; Treier, M.; Cai, J.; Aït-Mansour, K.; Ruffieux, P.; Gröning, O.; Gröning, P.; Kastler, M.; Rieger, R.; Feng, X.; et al. Porous graphenes: Two-dimensional polymer synthesis with atomic precision. Chem. Commun. 2009, 6919–6921. [Google Scholar] [CrossRef] [PubMed]
- Blunt, M.O.; Russell, J.C.; Champness, N.R.; Beton, P.H. Templating molecular adsorption using a covalent organic framework. Chem. Commun. 2010, 46, 7157–7159. [Google Scholar] [CrossRef]
- Sakamoto, J.; van Heijst, J.; Lukin, O.; Schluter, A.D. Two-dimensional polymers: Just a dream of synthetic chemists? Angew. Chem. Int. Ed. 2009, 48, 1030–1069. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Wang, Y.; Hahn, Y.-B. Graphene and its derivatives for solar cells application. Nano Energy 2018, 47, 51–65. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef]
- Velasco-Soto, M.A.; Pérez-Garcıa, S.A.; Alvarez-Quintana, J.; Cao, Y.; Nyborg, L.; Licea-Jiménez, L. Selective band gap manipulation of graphene oxide by its reduction with mild reagents. Carbon 2015, 93, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [Green Version]
- Stepanow, S.; Lingenfelder, M.; Dmitriev, A.; Spillmann, H.; Delvigne, E.; Lin, N.; Deng, X.; Cai, C.; Barth, J.V.; Kern, K. Steering molecular organization and host-guest interactions using two-dimensional nanoporous coordination systems. Nat. Mater. 2004, 3, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Bieri, M.; Nguyen, M.T.; Gröning, O.; Cai, J.M.; Treier, M.; Ait-Mansour, K.; Ruffieux, P.; Pignedoli, C.A.; Passerone, D.; Kastler, M.; et al. Two-dimensional polymer formation on surfaces: Insight into the roles of precursor mobility and reactivity. J. Am. Chem. Soc. 2010, 132, 16669–16676. [Google Scholar] [CrossRef] [PubMed]
- Ourdjini, O.; Pawlak, R.; Abel, M.; Clair, S.; Chen, L.; Bergeon, N.; Sassi, M.; Oison, V.; Debierre, J.-M.; Coratger, R.; et al. Substrate-mediated ordering and defect analysis of a surface covalent organic framework. Phys. Rev. B 2011, 84, 125421. [Google Scholar] [CrossRef]
- Shen, Q.; Gao, H.-Y.; Fuchs, H. Frontiers of on-surface synthesis: From principles to applications. Nano Today 2017, 13, 77–96. [Google Scholar] [CrossRef]
- Hu, J.; Liang, Z.; Shen, K.; Sun, H.; Jiang, Z.; Song, F. Recent progress in the fabrication of low dimensional nanostructures via surface-assisted transforming and coupling. J. Nanomater. 2017, 2017, 4796538. [Google Scholar] [CrossRef]
- Treier, M.; Pignedoli, C.A.; Laino, T.; Rieger, R.; Müllen, K.; Passerone, D.; Fasel, R. Surface-assisted cyclodehydrogenation provides a synthetic route towards easily processable and chemically tailored nanographenes. Nat. Chem. 2011, 3, 61–67. [Google Scholar] [CrossRef]
- Han, P.; Akagi, K.; Federici Canova, F.; Mutoh, H.; Shiraki, S.; Iwaya, K.; Weiss, P.S.; Asao, N.; Hitosugi, T. Bottom-up graphene-nanoribbon fabrication reveals chiral edges and enantioselectivity. ACS Nano 2014, 8, 9181–9187. [Google Scholar] [CrossRef]
- Rastgoo-Lahrood, A.; Macknapp, K.; Ritter, V.; Sotier, S.; Heckl, W.M.; Lackinger, M.; Spitzer, S. Solvent-free on-surface synthesis of boroxine COF monolayers. Chem. Commun. 2017, 53, 5147–5150. [Google Scholar] [CrossRef]
- Dienstmaier, J.F.; Medina, D.D.; Dogru, M.; Knochel, P.; Bein, T.; Heckl, W.M.; Lackinger, M. Isoreticular two-dimensional covalent organic frameworks synthesized by on-surface condensation of diboronic acids. ACS Nano 2012, 6, 7234–7242. [Google Scholar] [CrossRef]
- Dienstmaier, J.F.; Gigler, A.M.; Goetz, A.J.; Knochel, P.; Bein, T.; Lyapin, A.; Reichlmaier, S.; Heckl, W.M.; Lackinger, M. Synthesis of well-ordered COF monolayers: Surface growth of nanocrystalline precursors versus direct on-surface polycondensation. ACS Nano 2011, 5, 9737–9745. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Zeng, Q.; Lei, S. A photoresponsive surface covalent organic framework: Surface-confined synthesis, isomerization, and controlled guest capture and release. Chem. Eur. J. 2016, 22, 6768–6773. [Google Scholar] [CrossRef]
- Calik, M.; Sick, T.; Dogru, M.; Döblinger, M.; Datz, S.; Budde, H.; Hartschuh, A.; Auras, F.; Bein, T. From highly crystalline to outer surface-functionalized covalent organic frameworks: A modulation approach. J. Am. Chem. Soc. 2016, 138, 1234–1239. [Google Scholar] [CrossRef]
- Zwaneveld, N.A.A.; Pawlak, R.; Abel, M.; Catalin, D.; Gigmes, D.; Bertin, D.; Porte, L. Organized formation of 2D extended covalent organic frameworks at surfaces. J. Am. Chem. Soc. 2008, 130, 6678–6679. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.-B.; Wang, D. Construction of boronate ester based single-layered covalent organic frameworks. Chem. Commun. 2016, 52, 13771–13774. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, C.; Li, Z.; Kong, H.; Tan, Q.; Hu, A.; Xu, W. On-surface formation of one-dimensional polyphenylene through Bergman cyclization. J. Am. Chem. Soc. 2013, 135, 8448–8451. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-Y.; Wagner, H.; Zhong, D.; Franke, J.-H.; Studer, A.; Fuchs, H. Glaser coupling at metal surfaces. Angew. Chem. Int. Ed. 2013, 52, 4024–4028. [Google Scholar] [CrossRef] [PubMed]
- Klappenberger, F.; Hellwig, R.; Du, P.; Paintner, T.; Uphoff, M.; Zhang, L.; Lin, T.; Moghanaki, B.A.; Paszkiewicz, M.; Vobornik, I.; et al. Functionalized graphdiyne nanowires: On-surface synthesis and assessment of band structure, flexibility, and information storage potential. Small 2018, 14, 1704321. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cai, L.; Ding, Y.; Ma, H.; Yuan, C.; Xu, W. Single-molecule insight into Wurtz reactions on metal surfaces. Phys. Chem. Chem. Phys. 2016, 18, 2730–2735. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cai, L.; Ding, Y.; Xie, L.; Zhang, C.; Tan, Q.; Xu, W. Dehydrogenative homocoupling of terminal alkenes on copper surfaces: A route to dienes. Angew. Chem. Int. Ed. 2015, 54, 4549–4552. [Google Scholar] [CrossRef] [PubMed]
- Wiengarten, A.; Seufert, K.; Auwärter, W.; Ecija, D.; Diller, K.; Allegretti, F.; Bischoff, F.; Fischer, S.; Duncan, D.A.; Papageorgiou, A.C.; et al. Surface-assisted dehydrogenative homocoupling of porphine molecules. J. Am. Chem. Soc. 2014, 136, 9346–9354. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, C.; Kong, H.; Tan, Q.; Xu, W. On-surface aryl-aryl coupling via selective C-H activation. Chem. Commun. 2014, 50, 11825–11828. [Google Scholar] [CrossRef]
- Shu, C.-H.; Liu, M.-X.; Zha, Z.-Q.; Pan, J.-L.; Zhang, S.-Z.; Xie, Y.-L.; Chen, J.-L.; Yuan, D.-W.; Qiu, X.-H.; Liu, P.-N. On-surface synthesis of poly(p-phenylene ethynylene) molecular wires via in situ formation of carbon-carbon triple bond. Nat. Commun. 2018, 9, 2322. [Google Scholar] [CrossRef]
- Yang, B.; Björk, J.; Lin, H.; Zhang, X.; Zhang, H.; Li, Y.; Fan, J.; Li, Q.; Chi, L. Synthesis of surface covalent organic frameworks via dimerization and cyclotrimerization of acetyls. J. Am. Chem. Soc. 2015, 137, 4904–4907. [Google Scholar] [CrossRef]
- Weigelt, S.; Busse, C.; Bombis, C.; Knudsen, M.M.; Gothelf, K.V.; Strunskus, T.; Wöll, C.; Dahlbom, M.; Hammer, B.; Lægsgaard, E.; et al. Covalent interlinking of an aldehyde and an amine on a Au(111) surface in ultrahigh vacuum. Angew. Chem. Int. Ed. 2007, 46, 9227–9230. [Google Scholar] [CrossRef]
- Weigelt, S.; Busse, C.; Bombis, C.; Knudsen, M.M.; Gothelf, K.V.; Lægsgaard, E.; Besenbacher, F.; Linderoth, T.R. Surface synthesis of 2D branched polymer nanostructures. Angew. Chem. Int. Ed. 2008, 47, 4406–4410. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, R.; Higuchi, R.; Enoki, N.; Miyasato, Y.; Uemura, S.; Kimizuka, N.; Stieg, A.Z.; Gimzewski, J.K.; Kunitake, M. Thermodynamically controlled self-assembly of covalent nanoarchitectures in aqueous solution. ACS Nano 2011, 5, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Wan, J.H.; Deng, K.; Han, X.N.; Lei, S.B.; Yang, Y.L.; Zheng, Q.Y.; Zeng, Q.D.; Wang, C. Transformation of self-assembled structure by the addition of active reactant. J. Phys. Chem. C 2011, 115, 6540–6544. [Google Scholar] [CrossRef]
- Tanoue, R.; Higuchi, R.; Ikebe, K.; Uemura, S.; Kimizuka, N.; Stieg, A.Z.; Gimzewski, J.K.; Kunitake, M. In situ STM investigation of aromatic poly(azomethine) arrays constructed by “on-site” equilibrium polymerization. Langmuir 2012, 28, 13844–13851. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Guan, C.Z.; Ding, S.Y.; Wang, W.; Yan, H.J.; Wang, D.; Wan, L.J. On-surface synthesis of single-layered two-dimensional covalent organic frameworks via solid-vapor interface reactions. J. Am. Chem. Soc. 2013, 135, 10470–10474. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Y.; Zhang, X.M.; Wang, X.; Wang, S.; Wang, H.Q.; Duan, W.B.; Zeng, Q.D.; Wang, C. In situ STM investigation of two-dimensional chiral assemblies through Schiff-base condensation at a liquid/solid interface. ACS Appl. Mater. Interf. 2013, 5, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.R.; Zhou, X.; Yu, Y.X.; Tian, W.Q.; Ma, J.; Lei, S.B. Surface-confined crystalline two-dimensional covalent organic frameworks. ACS Nano 2013, 7, 8066–8073. [Google Scholar] [CrossRef] [PubMed]
- Bisbey, R.P.; Dichtel, W.R. Covalent organic frameworks as a platform for multidimensional polymerization. ACS Cent. Sci. 2017, 3, 533–543. [Google Scholar] [CrossRef]
- Marele, A.C.; Mas-Ballesté, R.; Terracciano, L.; Rodríguez-Fernández, J.; Berlanga, I.; Alexandre, S.S.; Otero, R.; Gallego, J.M.; Zamora, F.; Gómez-Rodríguez, J.M. Formation of a surface covalent organic framework based on polyester condensation. Chem. Commun. 2012, 48, 6779–6781. [Google Scholar] [CrossRef] [PubMed]
- Treier, M.; Richardson, N.V.; Fasel, R. Fabrication of surface-supported low-dimensional polyimide networks. J. Am. Chem. Soc. 2008, 130, 14054–14055. [Google Scholar] [CrossRef] [PubMed]
- Treier, M.; Fasel, R.; Champness, N.R.; Argent, S.; Richardson, N.V. Molecular imaging of polyimide formation. Phys. Chem. Chem. Phys. 2009, 11, 1209–1214. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Greenwood, J.; Früchtl, H.A.; Baddeley, C.J. STM investigation on the formation of oligoamides on Au{111} by surface-confined reactions of melamine with trimesoyl chloride. J. Phys. Chem. C 2011, 115, 8630–8636. [Google Scholar] [CrossRef]
- Schmitz, C.H.; Ikonomov, J.; Sokolowski, M. Two-dimensional polyamide networks with a broad pore size distribution on the Ag(111) surface. J. Phys. Chem. C 2011, 115, 7270–7278. [Google Scholar] [CrossRef]
- Wäckerlin, C.; Li, J.; Mairena, A.; Martin, K.; Avarvari, N.; Ernst, K.-H. Surface-assisted diastereoselective Ullmann coupling of bishelicenes. Chem. Commun. 2016, 52, 12694–12697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalashnyk, N.; Mouhat, K.; Oh, J.; Jung, J.; Xie, Y.; Giovanelli, L.; Salomon, E.; Angot, T.; Dumur, F.; Gigmes, D.; Clair, S. On-surface synthesis of aligned functional nanoribbons monitored by vibrational spectroscopy. Nature Commun. 2017, 8, 14735. [Google Scholar] [CrossRef] [PubMed]
- Kalashnyk, N.; Salomon, E.; Mun, S.H.; Jung, J.; Giovanelli, L.; Angot, T.; Dumur, F.; Gigmes, D.; Clair, S. The orientation of silver surfaces drives the reactivity and the selectivity in homo-coupling reactions. ChemPhysChem 2018, 19, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Kalashnyk, N.; Dumur, F.; Gigmes, D.; Clair, S. Molecular adaptation in supra-molecular self-assembly: Brickwall-type phases of indacene-tetrone on silver surfaces. Chem. Commun. 2018, 54, 8510–8513. [Google Scholar] [CrossRef]
- Zhong, D.; Franke, J.-H.; Podiyanachari, S.K.; Blömker, T.; Zhang, H.; Kehr, G.; Erker, G.; Fuchs, H.; Chi, L. Linear alkane polymerization on a gold surface. Science 2011, 334, 213–216. [Google Scholar] [CrossRef]
- In’t Veld, M.; Iavicoli, P.; Haq, S.; Amabilino, D.B.; Raval, R. Unique intermolecular reaction of simple porphyrins at a metal surface gives covalent nanostructures. Chem. Commun. 2008, 1536–1538. [Google Scholar] [CrossRef]
- Held, P.A.; Fuchs, H.; Studer, A. Covalent-bond formation via on-surface chemistry. Chem. Eur. J. 2017, 23, 5874–5892. [Google Scholar] [CrossRef]
- Dienel, T.; Gomez-Dıaz, J.; Seitsonen, A.P.; Widmer, R.; Iannuzzi, M.; Radican, K.; Sachdev, H.; Mullen, K.; Hutter, J.; Groning, O. Dehalogenation and coupling of a polycyclic hydrocarbon on an atomically thin insulator. ACS Nano 2014, 8, 6571–6579. [Google Scholar] [CrossRef]
- Killops, K.L.; Campos, L.M.; Hawker, C.J. Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene “Click” chemistry. J. Am. Chem. Soc. 2008, 130, 5062–5064. [Google Scholar] [CrossRef]
- Fickert, J.; Makowski, M.; Kappl, M.; Landfester, K.; Crespy, D. Efficient encapsulation of self-healing agents in polymer nanocontainers functionalized by orthogonal reactions. Macromolecules 2012, 45, 6324–6332. [Google Scholar] [CrossRef]
- Joralemon, M.J.; O’Reilly, R.K.; Hawker, C.J.; Wooley, K.L. Shell click-crosslinked (SCC) nanoparticles: A new methodology for synthesis and orthogonal functionalization. J. Am. Chem. Soc. 2005, 127, 16892–16899. [Google Scholar] [CrossRef]
- Zeng, Y.-F.; Zou, R.-Y.; Luo, Z.; Zhang, H.-C.; Yao, X.; Ma, X.; Zou, R.-Q.; Zhao, Y.-L. Covalent organic frameworks formed with two types of covalent bonds based on orthogonal reactions. J. Am. Chem. Soc. 2015, 137, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Addicoat, M.; Jin, E.-Q.; Xu, H.; Hayashi, T.; Xu, F.; Huang, N.; Irle, S.; Jiang, D.-L. Designed synthesis of double-stage two-dimensional covalent organic frameworks. Sci. Rep. 2015, 5, 14650–14668. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.; Vilas-Varela, M.; Kretz, B.; Garcia-Lekue, A.; Costache, M.V.; Paradinas, M.; Panighel, M.; Ceballos, G.; Valenzuela, S.O.; Peña, D.; et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 2018, 360, 199–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Joshi, T.; Li, H.; Chavez, A.D.; Pedramrazi, Z.; Liu, P.-N.; Li, H.; Dichtel, W.R.; Bredas, J.-L.; Crommie, M.F. Local electronic structure of a single-layer porphyrin-containing covalent organic framework. ACS Nano 2018, 12, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Oses, M.; de Oteyza, D.G.; Fernandez-Torrente, I.; Gonzalez-Lakunza, N.; Schmidt-Weber, P.M.; Kampen, T.; Horn, K.; Gourdon, A.; Arnau, A.; Ortega, J.E. Non-covalent interactions in supramolecular assemblies investigated with electron spectroscopies. Chemphyschem 2009, 10, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, P.; Stepanow, S.; Dmitriev, A.; Honolka, J.; de Groot, F.M.F.; Lingenfelder, M.; Sen Gupta, S.; Sarma, D.D.; Bencok, P.; Stanescu, S.; et al. Supramolecular control of the magnetic anisotropy in two-dimensional high-spin Fe arrays at a metal interface. Nat. Mater. 2009, 8, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakirov, S. Condensation polymers: Their chemical peculiarities offer great opportunities. Progr. Polym. Sci. 2019, 89, 1–18. [Google Scholar] [CrossRef]
- Lipton-Duffin, J.A.; Ivasenko, O.; Perepichka, D.F.; Rosei, F. Synthesis of polyphenylene molecular wires by surface-confined polymerization. Small 2009, 5, 592–597. [Google Scholar] [CrossRef]
- Lafferentz, L.; Eberhardt, V.; Dri, C.; Africh, C.; Comelli, G.; Esch, F.; Hecht, S.; Grill, L. Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 2012, 4, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, S.; Heckl, W.M.; Lackinger, M. On-surface radical addition of triply iodinated monomers on Au(111): The influence of monomer size and thermal post-processing. Surf. Sci. 2012, 606, 999–1004. [Google Scholar] [CrossRef]
- Walch, H.; Gutzler, R.; Sirtl, T.; Eder, G.; Lackinger, M. Material- and orientation-dependent reactivity for heterogeneously catalyzed carbon-bromine bond homolysis. J. Phys. Chem. C 2010, 114, 12604–12609. [Google Scholar] [CrossRef]
- Gutzler, R.; Walch, H.; Eder, G.; Kloft, S.; Heckl, W.M.; Lackinger, M. Surface mediated synthesis of 2D covalent organic frameworks: 1,3,5-tris(4-bromophenyl)benzene on Graphite(001), Cu(111), and Ag(110). Chem. Commun. 2009, 4456–4458. [Google Scholar] [CrossRef] [PubMed]
- Gutzler, R.; Cardenas, L.; Lipton-Duffin, J.; El Garah, M.; Dinca, L.E.; Szakacs, C.E.; Fu, C.; Gallagher, M.; Vondracek, M.; Rybachuk, M.; et al. Ullmann-type coupling of brominated tetrathienoanthracene on copper and silver. Nanoscale 2014, 6, 2660–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichhorn, J.; Strunskus, T.; Rastgoo-Lahrood, A.; Samanta, D.; Schmittel, M.; Lackinger, M. On-surface Ullmann polymerization via intermediate organometallic networks on Ag(111). Chem. Commun. 2014, 50, 7680–7682. [Google Scholar] [CrossRef] [Green Version]
- Eichhorn, J.; Nieckarz, D.; Ochs, O.; Samanta, D.; Schmittel, M.; Szabelski, P.J.; Lackinger, M. On-surface Ullmann coupling: The influence of kinetic reaction parameters on the morphology and quality of covalent networks. ACS Nano 2014, 8, 7880–7889. [Google Scholar] [CrossRef]
- Shi, K.J.; Zhang, X.; Shu, C.H.; Li, D.Y.; Wu, X.Y.; Liu, P.N. Ullmann coupling reaction of aryl chlorides on Au(111) using dosed Cu as a catalyst and the programmed growth of 2D covalent organic frameworks. Chem. Commun. 2016, 52, 8726–8729. [Google Scholar] [CrossRef]
- Peyrot, D.; Silly, M.G.; Silly, F. Temperature-triggered sequential on-surface synthesis of one and two covalently bonded porous organic nanoarchitectures on Au(111). J. Phys. Chem. C 2017, 121, 26815–26821. [Google Scholar] [CrossRef]
- Guan, C.Z.; Wang, D.; Wan, L.J. Construction and repair of highly ordered 2D covalent networks by chemical equilibrium regulation. Chem. Commun. 2012, 48, 2943–2945. [Google Scholar] [CrossRef] [PubMed]
- Stredansky, M.; Sala, A.; Fontanot, T.; Costantini, R.; Africh, C.; Comelli, G.; Floreano, L.; Morgante, A.; Cossaro, A. On-surface synthesis of a 2D boroxine framework: A route to a novel 2D material? Chem. Commun. 2018, 54, 3971–3973. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, S.; Sirtl, T.; Eichhorn, J.; Heckl, W.M.; Lackinger, M. Synthesis of two-dimensional phenylene–boroxine networks through in vacuo condensation and on-surface radical addition. Chem. Commun. 2011, 47, 12355–12357. [Google Scholar] [CrossRef]
- Faury, T.; Clair, S.; Abel, M.; Dumur, F.; Gigmes, D.; Porte, L. Sequential linking to control growth of a surface covalent organic framework. J. Phys. Chem. C 2012, 116, 4819–4823. [Google Scholar] [CrossRef]
- Clair, S.; Ourdjini, O.; Abel, M.; Porte, L. Tip- or electron beam-induced surface polymerization. Chem. Commun. 2011, 47, 8028–8030. [Google Scholar] [CrossRef]
- Yue, J.-Y.; Mo, Y.-P.; Li, S.-Y.; Dong, W.-L.; Chen, T.; Wang, D. Simultaneous construction of two linkages for the on-surface synthesis of imine–boroxine hybrid covalent organic frameworks. Chem. Sci. 2017, 8, 2169–2174. [Google Scholar] [CrossRef] [Green Version]
- Koudia, M.; Abel, M. Step-by-step on-surface synthesis: From manganese phthalocyanines to their polymeric form. Chem. Commun. 2014, 50, 8565–8567. [Google Scholar] [CrossRef]
- Gottfried, J.M. Surface chemistry of porphyrins and phthalocyanines. Surf. Sci. Rep. 2015, 70, 259–379. [Google Scholar] [CrossRef]
- Abel, M.; Clair, S.; Ourdjini, O.; Mossoyan, M.; Porte, L. Single layer of polymeric Fe-phthalocyanine: An organometallic sheet on metal and thin insulating film. J. Am. Chem. Soc. 2011, 133, 1203–1205. [Google Scholar] [CrossRef]
- Cai, J.; Ruffieux, P.; Jaafar, R.; Bieri, M.; Braun, T.; Blankenburg, S.; Muoth, M.; Seitsonen, A.P.; Saleh, M.; Feng, X.; et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 2010, 466, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Teeter, J.D.; Costa, P.S.; Zahl, P.; Vo, T.H.; Shekhirev, M.; Xu, W.; Zeng, X.C.; Enders, A.; Sinitskii, A. Dense monolayer films of atomically precise graphene nanoribbons on metallic substrates enabled by direct contact transfer of molecular precursors. Nanoscale 2017, 9, 18835–18844. [Google Scholar] [CrossRef] [PubMed]
- Kimouche, A.; Ervasti, M.M.; Drost, R.; Halonen, S.; Harju, A.; Joensuu, P.M.; Sainio, J.; Liljeroth, P. Ultra-narrow metallic armchair graphene nanoribbons. Nat. Commun. 2015, 6, 10177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdurakhmanova, N.; Amsharov, N.; Stepanow, S.; Jansen, M.; Kern, K.; Amsharov, K. Synthesis of wide atomically precise graphene nanoribbons from para-oligophenylene based molecular precursor. Carbon 2014, 77, 1187–1190. [Google Scholar] [CrossRef]
- Han, P.; Akagi, K.; Federici Canova, F.; Shimizu, R.; Oguchi, H.; Shiraki, S.; Weiss, P.S.; Asao, N.; Hitosugi, T. Self-assembly strategy for fabricating connected graphene nanoribbons. ACS Nano 2015, 9, 12035–12044. [Google Scholar] [CrossRef] [PubMed]
- Ruffieux, P.; Wang, S.; Yang, B.; Sánchez-Sánchez, C.; Liu, J.; Dienel, T.; Talirz, L.; Shinde, P.; Pignedoli, C.A.; Passerone, D.; et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 2016, 531, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonov, K.A.; Vinogradov, N.A.; Vinogradov, A.S.; Generalov, A.V.; Zagrebina, E.M.; Svirskiy, G.I.; Cafolla, A.A.; Carpy, T.; Cunniffe, J.P.; Taketsugu, T.; et al. From graphene nanoribbons on Cu(111) to nanographene on Cu(110): Critical role of substrate structure in the bottom-up fabrication strategy. ACS Nano 2015, 9, 8997–9011. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, C.; Dienel, T.; Deniz, O.; Ruffieux, P.; Berger, R.; Feng, X.; Müllen, K.; Fasel, R. Purely armchair or partially chiral: Noncontact atomic force microscopy characterization of dibromobianthryl-based graphene nanoribbons grown on Cu(111). ACS Nano 2016, 10, 8006–8011. [Google Scholar] [CrossRef] [PubMed]
- Talirz, L.; Söde, H.; Dumslaff, T.; Wang, S.; Sanchez-Valencia, J.R.; Liu, J.; Shinde, P.; Pignedoli, C.A.; Liang, L.; Meunier, V.; et al. On-surface synthesis and characterization of 9-atom wide armchair graphene nanoribbons. ACS Nano 2017, 11, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Teeter, J.D.; Costa, P.S.; Mehdi Pour, M.; Miller, D.P.; Zurek, E.; Enders, A.; Sinitskii, A. Epitaxial growth of aligned atomically precise chevron graphene nanoribbons on Cu(111). Chem. Commun. 2017, 53, 8463–8466. [Google Scholar] [CrossRef] [PubMed]
- Bronner, C.; Stremlau, S.; Gille, M.; Brauße, F.; Haase, A.; Hecht, S.; Tegeder, P. Aligning the band gap of graphene nanoribbons by monomer doping. Angew. Chem. Int. Ed. 2013, 52, 4422–4425. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.H.; Perera, U.G.E.; Shekhirev, M.; Mehdi Pour, M.; Kunkel, D.A.; Lu, H.; Gruverman, A.; Sutter, E.; Cotlet, M.; Nykypanchuk, D.; et al. Nitrogen-doping induced self-assembly of graphene nanoribbon-based two-dimensional and three-dimensional metamaterials. Nano Lett. 2015, 15, 5770–5777. [Google Scholar] [CrossRef]
- Cloke, R.R.; Marangoni, T.; Nguyen, G.D.; Joshi, T.; Rizzo, D.J.; Bronner, C.; Cao, T.; Louie, S.G.; Crommie, M.F.; Fischer, F.R. Site-specific substitutional boron doping of semiconducting armchair graphene nanoribbons. J. Am. Chem. Soc. 2015, 137, 8872–8875. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Saito, S.; Osumi, S.; Yamaguchi, S.; Foster, A.S.; Spijker, P.; Meyer, E. Atomically controlled substitutional boron-doping of graphene nanoribbons. Nat. Commun. 2015, 6, 8098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marangoni, T.; Haberer, D.; Rizzo, D.J.; Cloke, R.R.; Fischer, F.R. Heterostructures through divergent edge reconstruction in nitrogen-doped segmented graphene nanoribbons. Chem. Eur. J. 2016, 22, 13037–13040. [Google Scholar] [CrossRef]
- Cai, J.; Pignedoli, C.A.; Talirz, L.; Ruffieux, P.; Soede, H.; Liang, L.; Meunier, V.; Berger, R.; Li, R.; Feng, X.; et al. Graphene nanoribbon heterojunctions. Nat. Nanotechnol. 2014, 9, 896–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-C.; Cao, T.; Chen, C.; Pedramrazi, Z.; Haberer, D.; de Oteyza, D.G.; Fischer, F.R.; Louie, S.G.; Crommie, M.F. Molecular bandgap engineering of bottom up synthesized graphene nanoribbon heterojunctions. Nat. Nanotechnol. 2015, 10, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, H.; Sun, K.; Chen, L.; Zagranyarski, Y.; Aghdassi, N.; Duhm, S.; Li, Q.; Zhong, D.; Li, Y.; et al. On-surface synthesis of rylene-type graphene nanoribbons. J. Am. Chem. Soc. 2015, 137, 4022–4025. [Google Scholar] [CrossRef] [PubMed]
- de Oteyza, D.G.; García-Lekue, A.; Vilas-Varela, M.; Merino-Díez, N.; Carbonell-Sanromà, E.; Corso, M.; Vasseur, G.; Rogero, C.; Guitián, E.; Pascual, J.I.; et al. Substrate-independent growth of atomically precise chiral graphene nanoribbons. ACS Nano 2016, 10, 9000–9008. [Google Scholar] [CrossRef] [PubMed]
- Creencia, E.C.; Horaguchi, T. Thermal decomposition reactions of n-alkylated 2-aminobiphenyls to carbazole and phenanthridine. J. Heterocycl. Chem. 2006, 43, 1441–1446. [Google Scholar] [CrossRef]
- Schulz, F.; Jacobse, P.H.; Canova, F.F.; van der Lit, J.; Gao, D.Z.; van den Hoogenband, A.; Han, P.; Klein Gebbink, R.J.M.; Moret, M.-E.; Joensuu, P.M.; et al. Precursor geometry determines the growth mechanism in graphene nanoribbons. J. Phys. Chem. C 2017, 121, 2896–2904. [Google Scholar] [CrossRef]
- Simonov, K.A.; Vinogradov, N.A.; Vinogradov, A.S.; Generalov, A.V.; Zagrebina, E.M.; Mårtensson, N.; Cafolla, A.A.; Carpy, T.; Cunniffe, J.P.; Preobrajenski, A.B. Comment on “Bottom-up graphene-nanoribbon fabrication reveals chiral edges and enantioselectivity”. ACS Nano 2015, 9, 3399–3403. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Akagi, K.; Federici Canova, F.; Mutoh, H.; Shiraki, S.; Iwaya, K.; Weiss, P.S.; Asao, N.; Hitosugi, T. Reply to “Comment on ‘Bottom-up graphene-nanoribbon fabrication reveals chiral edges and enantioselectivity’”. ACS Nano 2015, 9, 3404–3405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Q.; He, Q.; Zhang, Y.; Fu, X.; Wang, X.; Zhao, D.; Chen, W.; Xu, G.Q.; Wu, K. Bromine adatom promoted C–H bond activation in terminal alkynes at room temperature on Ag(111). Phys. Chem. Chem. Phys. 2018, 20, 11081–11088. [Google Scholar] [CrossRef] [PubMed]
- Held, P.A.; Gao, H.-Y.; Liu, L.; Mîck-Lichtenfeld, C.; Timmer, A.; Mönig, H.; Barton, D.; Neugebauer, J.; Fuchs, H.; Studer, A. On-surface domino reactions: Glaser coupling and dehydrogenative coupling of a biscarboxylic acid to form polymeric bisacylperoxides. Angew. Chem. Int. Ed. 2016, 55, 9777–9782. [Google Scholar] [CrossRef] [PubMed]
- Clair, S.; Pons, S.; Seitsonen, A.P.; Brune, H.; Kern, K.; Barth, J.V. STM study of terephthalic acid self-assembly on Au(111): Hydrogen-bonded sheets on an inhomogeneous substrate. J. Phys. Chem. B 2004, 108, 14585–14590. [Google Scholar] [CrossRef]
- Payer, D.; Comisso, A.; Dmitriev, A.; Strunskus, T.; Lin, N.; Wöll, C.; DeVita, A.; Barth, J.V.; Kern, K. Ionic hydrogen bonds controlling two-dimensional supramolecular systems at a metal surface. Chem. Eur. J. 2007, 13, 3900–3906. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.; Lin, N.; Weckesser, J.; Barth, J.V.; Kern, K. Supramolecular assemblies of trimesic acid on a Cu(100) Surface. J. Phys. Chem. B 2002, 106, 6907–6912. [Google Scholar] [CrossRef]
- Gao, H.-Y.; Held, P.A.; Knor, M.; Mîck-Lichtenfeld, C.; Neugebauer, J.; Studer, A.; Fuchs, H. Decarboxylative polymerization of 2,6-naphthalenedicarboxylic acid at surfaces. J. Am. Chem. Soc. 2014, 136, 9658–9663. [Google Scholar] [CrossRef] [PubMed]
























© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigot, C.; Dumur, F. Recent Advances of Hierarchical and Sequential Growth of Macromolecular Organic Structures on Surface. Materials 2019, 12, 662. https://doi.org/10.3390/ma12040662
Pigot C, Dumur F. Recent Advances of Hierarchical and Sequential Growth of Macromolecular Organic Structures on Surface. Materials. 2019; 12(4):662. https://doi.org/10.3390/ma12040662
Chicago/Turabian StylePigot, Corentin, and Frédéric Dumur. 2019. "Recent Advances of Hierarchical and Sequential Growth of Macromolecular Organic Structures on Surface" Materials 12, no. 4: 662. https://doi.org/10.3390/ma12040662
APA StylePigot, C., & Dumur, F. (2019). Recent Advances of Hierarchical and Sequential Growth of Macromolecular Organic Structures on Surface. Materials, 12(4), 662. https://doi.org/10.3390/ma12040662