Cell Viability of Porous Poly(d,l-lactic acid)/Vertically Aligned Carbon Nanotubes/Nanohydroxyapatite Scaffolds for Osteochondral Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Superhydrophilic Vertically Aligned Multi-Walled Carbon Nanotubes Films (VAMWCNT-O)
2.2. Electrodeposition of nHA Crystals on the VAMWCNT-O (nHAp1)
2.3. Deposition of nHA Crystals on the VAMWCNT-O Using Simulated Body Fluid (nHAp2)
2.4. Production of Porous PDLLA/VAMWCNT-O:nHAp Scaffolds
2.5. Characterization of Porous PDLLA/VAMWCNT-O:nHAp Membranes
2.6. Cell Culture Experiments and Analysis
2.7. Cell Adhesion and Viability of PDLLA/VAMWCNT-O:nHAp Membranes
2.8. Cytotoxicity Assay
2.9. Gene Expression Analysis
2.10. Statistics Analysis
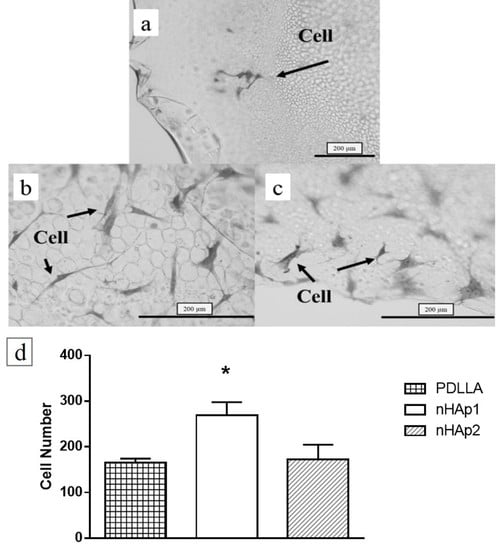
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernhard, J.C.; Vunjak-Novakovic, G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res. Ther. 2016, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-P.; Oliveira, J.M.; Oliveira, A.L.; Reis, R.L. Current Concepts and Challenges in Osteochondral Tissue Engineering and Regenerative Medicine. ACS Biomater. Sci. Eng. 2015, 1, 183–200. [Google Scholar] [CrossRef] [Green Version]
- Kon, E.; Filardo, G.; Perdisa, F.; Venieri, G.; Marcacci, M. Clinical results of multilayered biomaterials for osteochondral regeneration. J. Exp. Orthop. 2014, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Schofield, D.; Callander, E. The individual and socioeconomic impact of osteoarthritis. Nat. Rev. Rheumatol. 2014, 10, 437–441. [Google Scholar] [CrossRef]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019, 87, 41–54. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef] [PubMed]
- van Bergen, C.J.A.; Kox, L.S.; Maas, M.; Sierevelt, I.N.; Kerkhoffs, G.M.M.J.; van Dijk, C.N. Arthroscopic Treatment of Osteochondral Defects of the Talus. J. Bone Jt. Surg. 2013, 95, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Ulstein, S.; Årøen, A.; Engebretsen, L.; Forssblad, M.; Lygre, S.H.L.; Røtterud, J.H. A Controlled Comparison of Microfracture, Debridement, and No Treatment of Concomitant Full-Thickness Cartilage Lesions in Anterior Cruciate Ligament–Reconstructed Knees: A Nationwide Prospective Cohort Study From Norway and Sweden of 368 Patients With 5-Year Follow-up. Orthop. J. Sports Med. 2018, 6. [Google Scholar] [CrossRef]
- Brix, M.; Kaipel, M.; Kellner, R.; Schreiner, M.; Apprich, S.; Boszotta, H.; Windhager, R.; Domayer, S.; Trattnig, S. Successful osteoconduction but limited cartilage tissue quality following osteochondral repair by a cell-free multilayered nano-composite scaffold at the knee. Int. Orthop. 2016, 40, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.; Gracitelli, G.C.; Shah, N.; Chang, E.Y.; Gomoll, A.H. High Failure Rate of a Decellularized Osteochondral Allograft for the Treatment of Cartilage Lesions. Am. J. Sports Med. 2016, 44, 2015–2022. [Google Scholar] [CrossRef]
- Di Luca, A.; Longoni, A.; Criscenti, G.; Lorenzo-Moldero, I.; Klein-Gunnewiek, M.; Vancso, J.; Van Blitterswijk, C.; Mota, C.; Moroni, L. Surface energy and stiffness discrete gradients in additive manufactured scaffolds for osteochondral regeneration. Biofabrication 2016, 8, 15014. [Google Scholar] [CrossRef] [PubMed]
- Avitzour, M.; Mintz, Y.; Liebergall, M.; Mosheiff, R. The burden of terrorism: High rate of recurrent hospital referrals. Injury 2008, 39, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Wu, H.; Wan, Y.; Cao, X.; Wu, Q. Proliferation of chondrocytes on porous poly(dl-lactide)/chitosan scaffolds. Acta Biomater. 2008, 4, 76–87. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Denes, E.; Barrière, G.; Poli, E.; Lévêque, G. Commentary: Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2017, 5, 15. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicciù, M. Real Opportunity for the Present and a Forward Step for the Future of Bone Tissue Engineering. J. Craniofac. Surg. 2017, 28, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Herford, A.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar Ridge Augmentation with Titanium Mesh. A Retrospective Clinical Study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, J.; Wang, J.; Zhuang, X.; Chen, X. Biomimetic biphasic scaffolds for osteochondral defect repair Biomimetic biphasic scaffolds for osteochondral defect repair. Regen. Biomater. 2015, 2, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, K.; Moriguchi, Y.; Murawski, C.D.; Yoshikawa, H.; Nakamura, N. Osteochondral Tissue Engineering with Biphasic Scaffold: Current Strategies and Techniques. Tissue Eng. Part B Rev. 2014, 20, 468–476. [Google Scholar] [CrossRef]
- Yao, Q.; Nooeaid, P.; Detsch, R.; Roether, J.A.; Dong, Y.; Goudouri, O.M.; Schubert, D.W.; Boccaccini, A.R. Bioglass®/chitosan-polycaprolactone bilayered composite scaffolds intended for osteochondral tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 4510–4518. [Google Scholar] [CrossRef]
- Yunos, D.M.; Ahmad, Z.; Salih, V.; Boccaccini, A.R. Stratified scaffolds for osteochondral tissue engineering applications: Electrospun PDLLA nanofibre coated Bioglass®-derived foams. J. Biomater. Appl. 2013, 27, 537–551. [Google Scholar] [CrossRef]
- Lv, Y.M.; Yu, Q.S. Repair of articular osteochondral defects of the knee joint using a composite lamellar scaffold. Bone Jt. Res. 2015, 4, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-H.; Shen, B.-Y.; Wang, Y.-H.; Lin, B.; Lee, H.-M.; Hsieh, M.-F. Healing of Osteochondral Defects Implanted with Biomimetic Scaffolds of Poly(ε-Caprolactone)/Hydroxyapatite and Glycidyl-Methacrylate-Modified Hyaluronic Acid in a Minipig. Int. J. Mol. Sci. 2018, 19, 1125. [Google Scholar] [CrossRef] [PubMed]
- Grigolo, B.; Cavallo, C.; Desando, G.; Manferdini, C.; Lisignoli, G.; Ferrari, A.; Zini, N.; Facchini, A. Novel nano-composite biomimetic biomaterial allows chondrogenic and osteogenic differentiation of bone marrow concentrate derived cells. J. Mater. Sci. Mater. Med. 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zheng, Q.; Zong, C.; Li, Q.; Li, H.; Qian, S.; Zhang, B.; Yu, L.; Pan, Z. Osteochondral repair using porous poly(lactide-co-glycolide)/ nano-hydroxyapatite hybrid scaffolds with undifferentiated mesenchymal stem cells in a rat model. J. Biomed. Mater. Res. Part A 2010, 94, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Zuo, Y.; Qu, D.; Liu, Y.; Chen, T.; Jiang, N.; Li, H.; Li, J. Osteogenesis and chondrogenesis of biomimetic integrated porous PVA/gel/V-n-HA/pa6 scaffolds and BMSCs construct in repair of articular osteochondral defect. J. Biomed. Mater. Res. Part A 2015, 103, 3226–3236. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; Patel, R.; Zhang, L.G. Design of a Novel 3D Printed Bioactive Nanocomposite Scaffold for Improved Osteochondral Regeneration. Cell. Mol. Bioeng. 2015, 8, 416–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.P.; Silva-Correia, J.; Oliveira, M.B.; Vilela, C.; Pereira, H.; Sousa, R.A.; Mano, J.F.; Oliveira, A.L.; Oliveira, J.M.; Reis, R.L. Bilayered silk/silk-nanoCaP scaffolds for osteochondral tissue engineering: In vitro and in vivo assessment of biological performance. Acta Biomater. 2015, 12, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, B.V.M.; Silva, A.S.; Santana-Melo, G.; Vasconscellos, L.M.R.; Lobo, A.D.O.; Marciano, F.R. Influence of low contents of superhydrophilic MWCNT on the properties and cell viability of electrospun poly (butylene adipate-co-terephthalate) fibers. Mater. Sci. Eng. C 2016, 59, 782–791. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.G.; Rodrigues, B.V.M.; Ricci, R.; Costa, M.M.; Ribeiro, A.F.C.; Marciano, F.R.; Lobo, A.O. Designing a novel nanocomposite for bone tissue engineering using electrospun conductive PBAT/polypyrrole as a scaffold to direct nanohydroxyapatite electrodeposition. RSC Adv. 2016, 6, 32615–32623. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Liao, S.; Li, J. Carbon Nanotubes Reinforced Composites for Biomedical Applications. Biomed. Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Nano-Hydroxyapatite Composite Biomaterials for Bone Tissue Engineering—A Review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef]
- Siqueira, I.A.W.B.; Corat, M.A.F.; Cavalcanti, B.D.N.; Neto, W.A.R.; Martin, A.A.; Bretas, R.E.S.; Marciano, F.R.; Lobo, A.O. In vitro and in vivo studies of novel poly(d,l-lactic acid), superhydrophilic carbon nanotubes, and nanohydroxyapatite scaffolds for bone regeneration. ACS Appl. Mater. Interfaces 2015, 7, 9385–9398. [Google Scholar] [CrossRef]
- Lobo, A.O.; Marciano, F.R.; Ramos, S.C.; MacHado, M.M.; Corat, E.J.; Corat, M.A.F. Increasing mouse embryonic fibroblast cells adhesion on superhydrophilic vertically aligned carbon nanotube films. Mater. Sci. Eng. C 2011, 31, 1505–1511. [Google Scholar] [CrossRef]
- Lobo, A.O.; Corat, M.A.F.; Ramos, S.C.; Matsushima, J.T.; Granato, A.E.C.; Pacheco-Soares, C.; Corat, E.J. Fast preparation of hydroxyapatite/superhydrophilic vertically aligned multiwalled carbon nanotube composites for bioactive application. Langmuir 2010, 26, 18308–18314. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; van Blitterswijk, C.; de Groot, K.; Layrolle, P. Nucleation of biomimetic Ca–P coatings on Ti6Al4V from a SBF×5 solution: Influence of magnesium. Biomaterials 2002, 23, 2211–2220. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.; de Groot, K.; Layrolle, P. Influence of ionic strength and carbonate on the Ca-P coating formation from SBF×5 solution. Biomaterials 2002, 23, 1921–1930. [Google Scholar] [CrossRef]
- Marsi, T.C.O.; Corat, M.A.F.; Machado, M.M.; Corat, E.J.; Marciano, F.R.; Lobo, A.O. Correlation and Comparison Between Thermodynamic Aspects and Cytocompatibility of Cells on Superhydrophobic and Superhydrophilic Vertically Aligned Carbon Nanotubes. Curr. Phys. Chem. 2013, 3, 155–165. [Google Scholar] [CrossRef]
- Cai, K.; Yao, K.; Lin, S.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Gao, L. Poly(d,l-lactic acid) surfaces modified by silk fibroin: Effects on the culture of osteoblast in vitro. Biomaterials 2002, 23, 1153–1160. [Google Scholar] [CrossRef]
- Zhang, L.F.; Yang, D.J.; Chen, H.C.; Sun, R.; Xu, L.; Xiong, Z.C.; Govender, T.; Xiong, C.D. An ionically crosslinked hydrogel containing vancomycin coating on a porous scaffold for drug delivery and cell culture. Int. J. Pharm. 2008, 353, 74–87. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, P.; Ren, T.; Gu, S.; Pan, K. Poly (d,l-lactide)/nano-hydroxyapatite composite scaffolds for bone tissue engineering and biocompatibility evaluation. J. Mater. Sci. Mater. Med. 2008, 19, 1075–1082. [Google Scholar] [CrossRef]
- Deplaine, H.; Lebourg, M.; Ripalda, P.; Vidaurre, A.; Sanz-Ramos, P.; Mora, G.; Prõsper, F.; Ochoa, I.; Doblaré, M.; Gõmez Ribelles, J.L.; et al. Biomimetic hydroxyapatite coating on pore walls improves osteointegration of poly(L-lactic acid) scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 173–186. [Google Scholar] [CrossRef]
- Ronca, A.; Ambrosio, L.; Grijpma, D.W. Preparation of designed poly(d,l-/lactide)/nanosized hydroxyapatite composite structures by stereolithography. Acta Biomater. 2013, 9, 5989–5996. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Chen, X.; Zhi, W.; Liu, Y.; Cui, W.; Hu, S.; Li, X. Promoted healing of femoral defects with in situ grown fibrous composites of hydroxyapatite and poly(dl-lactide). J. Biomed. Mater. Res. Part A 2012, 100, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Ren, J.; Gu, S. Preparation and characterization of porous PDLLA/HA composite foams by supercritical carbon dioxide technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.O.; Siqueira, I.A.W.B.; das Neves, M.F.; Marciano, F.R.; Corat, E.J.; Corat, M.A.F. In vitro and in vivo studies of a novel nanohydroxyapatite/superhydrophilic vertically aligned carbon nanotube nanocomposites. J. Mater. Sci. Mater. Med. 2013, 24, 1723–1732. [Google Scholar] [CrossRef]
- Girotto, D.; Urbani, S.; Brun, P.; Renier, D.; Barbucci, R.; Abatangelo, G. Tissue-specific gene expression in chondrocytes grown on three-dimensional hyaluronic acid scaffolds. Biomaterials 2003, 24, 3265–3275. [Google Scholar] [CrossRef]
- Sutradhar, B.C.; Hong, G.; Ge, Z.; Kim, G. Enhancement of chondrogenesis via co-culture of bovine chondrocytes with demineralized bone matrix in chitosan-alginate beads. J. Med. Biol. Eng. 2013, 33, 518–525. [Google Scholar] [CrossRef]
- Stenhamre, H.; Thorvaldsson, A.; Enochson, L.; Walkenström, P.; Lindahl, A.; Brittberg, M.; Gatenholm, P. Nanosized fibers’ effect on adult human articular chondrocytes behavior. Mater. Sci. Eng. C 2013, 33, 1539–1545. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Tallheden, T.; Karlsson, C.; Brunner, A.; van der Lee, J.; Hagg, R.; Tommasini, R.; Lindahl, A. Gene expression during redifferentiation of human articular chondrocytes. Osteoarthr. Cartil. 2004, 12, 525–535. [Google Scholar] [CrossRef] [Green Version]
- Benya, P.D.; Padilla, S.R.; Nimni, M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell 1978, 15, 1313–1321. [Google Scholar] [CrossRef]
- Randolph, M.A.; Anseth, K.; Yaremchuk, M.J. Tissue engineering of cartilage. Clin. Plast. Surg. 2003, 30, 519–537. [Google Scholar] [CrossRef]
- Cibere, J.; Thorne, A.; Kopec, J.A.; Singer, J.; Canvin, J.; Robinson, D.B.; Pope, J.; Hong, P.; Grant, E.; Lobanok, T.; et al. Glucosamine sulfate and cartilage type II collagen degradation in patients with knee osteoarthritis: Randomized discontinuation trial results employing biomarkers. J. Rheumatol. 2005, 32, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Li, W.-J.; Jiang, Y.J.; Tuan, R.S. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006, 12, 1775–1785. [Google Scholar] [CrossRef] [PubMed]
- Yamane, S.; Iwasaki, N.; Kasahara, Y.; Harada, K.; Majima, T.; Monde, K.; Nishimura, S.I.; Minami, A. Effect of pore size on in vitro cartilage formation using chitosan-based hyaluronic acid hybrid polymer fibers. J. Biomed. Mater. Res. Part A 2007, 81, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Hubbell, J.A. Surface Treatments of Polymers for Biocompatibility. Annu. Rev. Mater. Sci. 1996, 26, 365–394. [Google Scholar] [CrossRef]





| MMP-3 | sense 5-ATTCCATGGAGCCAGGCTTTC-3′ |
| anti-sense 5′CATTTGGGTCAAACTCCACTGTG-3′ | |
| Type I Collagen | sense 5-CCGCCGCTTCACCTACAGC-3′ |
| antisense 5-TTTGTATTCAATCACTGTCTTGCC-3′ | |
| Type II Collagen | sense 5-CCGAATAGCAGGTTCACGTACA-3′ |
| antisense 5-CGATAACAGTCTTGCCCCACTT-3′ | |
| Aggrecan | sense 5-TTCAGTGGCCTACCAAGTGG-3′ |
| antisense 5-AGCCTGGGTTACAGATTCCA-3′ | |
| Sox-9 | sense 5-TGCTAGAAGATGAGGCTTCTGG-3′ |
| antisense 5-GGCACTTTGTCCAGACCCA-3′ | |
| Beta-actin | sense 5′-GGCACCCAGCACAATGAAG-3′ |
| antisense 5′-CCGATCCACACGGAGTACTTG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stocco, T.D.; Antonioli, E.; Elias, C.d.M.V.; Rodrigues, B.V.M.; Siqueira, I.A.W.d.B.; Ferretti, M.; Marciano, F.R.; Lobo, A.O. Cell Viability of Porous Poly(d,l-lactic acid)/Vertically Aligned Carbon Nanotubes/Nanohydroxyapatite Scaffolds for Osteochondral Tissue Engineering. Materials 2019, 12, 849. https://doi.org/10.3390/ma12060849
Stocco TD, Antonioli E, Elias CdMV, Rodrigues BVM, Siqueira IAWdB, Ferretti M, Marciano FR, Lobo AO. Cell Viability of Porous Poly(d,l-lactic acid)/Vertically Aligned Carbon Nanotubes/Nanohydroxyapatite Scaffolds for Osteochondral Tissue Engineering. Materials. 2019; 12(6):849. https://doi.org/10.3390/ma12060849
Chicago/Turabian StyleStocco, Thiago Domingues, Eliane Antonioli, Conceição de Maria Vaz Elias, Bruno Vinícius Manzolli Rodrigues, Idália Aparecida Waltrick de Brito Siqueira, Mario Ferretti, Fernanda Roberta Marciano, and Anderson Oliveira Lobo. 2019. "Cell Viability of Porous Poly(d,l-lactic acid)/Vertically Aligned Carbon Nanotubes/Nanohydroxyapatite Scaffolds for Osteochondral Tissue Engineering" Materials 12, no. 6: 849. https://doi.org/10.3390/ma12060849
APA StyleStocco, T. D., Antonioli, E., Elias, C. d. M. V., Rodrigues, B. V. M., Siqueira, I. A. W. d. B., Ferretti, M., Marciano, F. R., & Lobo, A. O. (2019). Cell Viability of Porous Poly(d,l-lactic acid)/Vertically Aligned Carbon Nanotubes/Nanohydroxyapatite Scaffolds for Osteochondral Tissue Engineering. Materials, 12(6), 849. https://doi.org/10.3390/ma12060849