Antimicrobial Effects of Chemically Functionalized and/or Photo-Heated Nanoparticles
Abstract
:1. Antimicrobial Resistance
2. Nanomaterials as Antibacterial Agents

3. Theoretical Description of Induced Temperature Variations
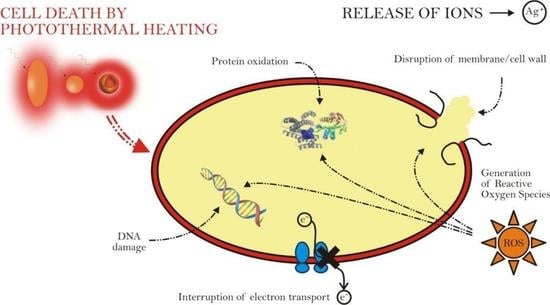
4. Anti-Microbial Effect of the Plasmonic Photothermal Heating of Gold Nanoparticles
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDR | multidrug resistence |
| EEA | European Economic Area |
| HGT | Horizontal gene transfer |
| NP | nanoparticle |
| GNP | gold nanoparticle |
| MNP | metal nanoparticle |
| LPR | localized plasmonic resonance |
| IR | infrared |
| NIR | near infrared |
References
- Theuretzbacher, U. Global antimicrobial resistance in gram-negative pathogens and clinical need. Curr. Opin. Microbiol. 2017, 39, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the eu and the european economic area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2018, 19, 56–66. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J.M. Acquired antibiotic resistance genes: An overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef] [PubMed]
- Salimiyan Rizi, K.K.; Noghondar, M. Adaptive antibiotic resistance: Overview and perspectives. J. Infect. Dis. Ther. 2018, 6, 1–3. [Google Scholar] [CrossRef]
- Juhas, M. Horizontal gene transfer in human pathogens. Crit. Rev. Microbiol. 2015, 41, 101–108. [Google Scholar] [CrossRef]
- Chen, J.; Quiles-Puchalt, N.; Chiang, Y.N.; Bacigalupe, R.; Fillol-Salom, A.; Chee, M.S.J.; Fitzgerald, J.R.; Penadés, J.R. Genome hypermobility by lateral transduction. Science 2018, 362, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.; Arias, C. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Moghaddam, M.J.; Mirbagheri, A.; Salehi, Z.; Habibzade, S. Prevalence of class 1 integrons and extended spectrum beta lactamases among multi-drug resistant Escherichia coli isolates from north of Iran. Iran. Biomed. J. 2015, 19, 233–239. [Google Scholar]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Chadha, T. Bacterial biofilms: Survival mechanisms and antibiotic resistance. J. Bacteriol. Parasitol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Ainsa, J. The antibiotic paradox. How the misuse of antibiotics destroys their curative powers. (2nd edn). Stuart B. Levy. Int. Microbiol. 2002, 5, 155–156. [Google Scholar] [CrossRef]
- Tsuji, J.S.; Maynard, A.D.; Howard, P.C.; James, J.T.; Lam, C.W.; Warheit, D.B.; Santamaria, A.B. Research strategies for safety evaluation of nanomaterials, part iv: Risk assessment of nanoparticles. Toxicol. Sci. 2005, 89, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Kreilgaard, M. Influence of microemulsions on cutaneous drug delivery. Adv. Drug Deliv. Rev. 2002, 54, S77–S98. [Google Scholar] [CrossRef]
- Cross, S.E.; Innes, B.; Roberts, M.S.; Tsuzuki, T.; Robertson, T.A.; McCormick, P. Human skin penetration of sunscreen nanoparticles: In-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol. Physiol. 2007, 20, 148–154. [Google Scholar] [CrossRef]
- Zhang, L.W.; Yu, W.W.; Colvin, V.L.; Monteiro-Riviere, N.A. Biological interactions of quantum dot nanoparticles in skin and in human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2008, 228, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Zvyagin, A.V.; Zhao, X.; Gierden, A.; Sanchez, W.; Ross, J.A.; Roberts, M.S. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J. Biomed. Opt. 2008, 13, 064031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Ho, P.L.; Tong, E.; Wang, L.; Xu, B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; van de Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Yoon, K.; Byeon, J.H.; Park, J.; Hwang, J. Susceptibility constants of Escherichia coli and bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Auffan, M.; Achouak, W.; Rose, J.; Roncato, M.A.; Chanéac, C.; Waite, D.T.; Masion, A.; Woicik, J.C.; Wiesner, M.R.; Bottero, J.Y. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli. Environ. Sci. Technol. 2008, 42, 6730–6735. [Google Scholar] [CrossRef]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.M. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. physico-chemical insight of the cytotoxicity mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef]
- Banoee, M.; Seif, S.; Nazari, Z.E.; Jafari-Fesharaki, P.; Shahverdi, H.R.; Moballegh, A.; Moghaddam, K.M.; Shahverdi, A.R. Zno nanoparticles enhanced antibacterial activity of ciprofloxacin against staphylococcus aureus and Escherichia coli. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 557–561. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and zno water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Wu, C.; Wu, Q.; Li, J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 2005, 16, 1912. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Kronvall, G. Antimicrobial resistance 1979–2009 at karolinska hospital, Sweden: Normalized resistance interpretation during a 30-year follow-up on staphylococcus aureus and Escherichia coli resistance development. Apmis 2010, 118, 621–639. [Google Scholar] [CrossRef]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogoi, S.K.; Gopinath, P.; Paul, A.; Ramesh, A.; Ghosh, S.S.; Chattopadhyay, A. Green fluorescent protein-expressing Escherichia coli as a model system for investigating the antimicrobial activities of silver nanoparticles. Langmuir 2006, 22, 9322–9328. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Govorov, A.O.; Zhang, W.; Skeini, T.; Richardson, H.; Lee, J.; Kotov, N.A. Gold nanoparticle ensembles as heaters and actuators: Melting and collective plasmon resonances. Nanoscale Res. Lett. 2006, 1, 84–90. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (pptt) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted nanoparticles for cancer therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-enabled photothermal cancer therapy: Impending clinical impact. Accounts Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; De, M.; Rotello, V.M. Drug and gene delivery using gold nanoparticles. Nanobiotechnology 2007, 3, 40–45. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar] [CrossRef]
- Boyer, D.; Tamarat, P.; Maali, A.; Lounis, B.; Orrit, M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science 2002, 297, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Barsic, D.N.; Guichard, A.R.; Brongersma, M.L. Plasmon-assisted local temperature control to pattern individual semiconductor nanowires and carbon nanotubes. Nano Lett. 2007, 7, 3523–3527. [Google Scholar] [CrossRef] [PubMed]
- Baroud, C.N.; Delville, J.E.; Gallaire, F.; Wunenburger, R. Thermocapillary valve for droplet production and sorting. Phys. Rev. E 2007, 75, 046302. [Google Scholar] [CrossRef]
- Placido, T.; Fanizza, E.; Cosma, P.; Striccoli, M.; Curri, M.L.; Comparelli, R.; Agostiano, A. Electroactive layer-by-layer plasmonic architectures based on au nanorods. Langmuir 2014, 30, 2608–2618. [Google Scholar] [CrossRef]
- Ros, I.; Placido, T.; Amendola, V.; Marinzi, C.; Manfredi, N.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Abbotto, A.; Pedron, D.; et al. Sers properties of gold nanorods at resonance with molecular, transverse, and longitudinal plasmon excitations. Plasmonics 2014, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Carslaw, H.S.; Jaeger, J.C. Conduction of Heat in Solids, 2nd ed.; Clarendon Press: Oxford, UK, 1959. [Google Scholar]
- Pezzi, L.; de Sio, L.; Veltri, A.; Placido, T.; Palermo, G.; Comparelli, R.; Curri, M.L.; Agostiano, A.; Tabirian, N.; Umeton, C. Photo-thermal effects in gold nanoparticles dispersed in thermotropic nematic liquid crystals. Phys. Chem. Chem. Phys. 2015, 17, 20281–20287. [Google Scholar] [CrossRef]
- Lamri, G.; Veltri, A.; Aubard, J.; Adam, P.-M.; Felidj, N.; Baudrion, A.-L. Polarization-dependent strong coupling between silver nanorods and photochromic molecules. Beilstein J. Nanotechnol. 2018, 9, 2657–2664. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, V.; Pezzi, L.; Veltri, A.; de Luca, A. Resonant gain singularities in 1d and 3d metal/dielectric multilayered nanostructures. ACS Nano 2016, 11, 1012–1025. [Google Scholar] [CrossRef]
- Pezzi, L.; Palermo, G.; Veltri, A.; Cataldi, U.; Bürgi, T.; Ritacco, T.; Giocondo, M.; Umeton, C.; de Luca, A. Photo-thermal study of a layer of randomly distributed gold nanoparticles: From nano-localization to macro-scale effects. J. Phys. D Appl. Phys. 2017, 50, 435302. [Google Scholar] [CrossRef]
- De Sio, L.; Placido, T.; Serak, S.; Comparelli, R.; Tamborra, M.; Tabiryan, N.; Curri, M.L.; Bartolino, R.; Umeton, C.; Bunning, T. Nano-localized heating source for photonics and plasmonics. Adv. Opt. Mater. 2013, 1, 899–904. [Google Scholar] [CrossRef]
- De Sio, L.; Placido, T.; Comparelli, R.; Curri, M.L.; Striccoli, M.; Tabiryan, N.; Bunning, T.J. Next-generation thermo-plasmonic technologies and plasmonic nanoparticles in optoelectronics. Prog. Quantum Electron. 2015, 41, 23–70. [Google Scholar] [CrossRef]
- Norman, R.S.; Stone, J.W.; Gole, A.; Murphy, C.J.; Sabo-Attwood, T.L. Targeted photothermal lysis of the pathogenic bacteria, pseudomonas aeruginosa, with gold nanorods. Nano Lett. 2008, 8, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Martínez, J.C.; Martínez-Castañón, G.A.; Martínez-Gutierrez, F.; Zavala-Alonso, N.V.; Patiño-Marín, N.; Niño-Martinez, N. Antibacterial and antibiofilm activities of the photothermal therapy using gold nanorods against seven different bacterial strains. J. Nanomater. 2015, 16, 177. [Google Scholar] [CrossRef]
- Santos, G.M.; Ferrara, F.I.; Zhao, F.; Rodrigues, D.F.; Shih, W. Photothermal inactivation of heat-resistant bacteria on nanoporous gold disk arrays. Opt. Mater. Express 2016, 6, 1217–1229. [Google Scholar] [CrossRef]
- Teng, C.P.; Zhou, T.; Ye, E.; Liu, S.; Koh, L.D.; Low, M.; Loh, X.J.; Win, K.Y.; Zhang, L.; Han, M. Effective targeted photothermal ablation of multidrug resistant bacteria and their biofilms with nir-absorbing gold nanocrosses. Adv. Healthc. Mater. 2016, 5, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Pihl, M.; Bruzell, E.; Andersson, M. Bacterial biofilm elimination using gold nanorod localised surface plasmon resonance generated heat. Mater. Sci. Eng. C 2017, 80, 54–58. [Google Scholar] [CrossRef]
- Loeb, S.; Li, C.; Kim, J. Solar photothermal disinfection using broadband-light absorbing gold nanoparticles and carbon black. Environ. Sci. Technol. 2017, 52, 205–213. [Google Scholar] [CrossRef] [PubMed]
- De Sio, L.; Klein, G.; Serak, S.; Tabiryan, N.; Cunningham, A.; Tone, C.M.; Ciuchi, F.; Bürgi, T.; Umeton, C.; Bunning, T. All-optical control of localized plasmonic resonance realized by photoalignment of liquid crystals. J. Mater. Chem. C 2013, 1, 7483–7487. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pezzi, L.; Pane, A.; Annesi, F.; Losso, M.A.; Guglielmelli, A.; Umeton, C.; De Sio, L. Antimicrobial Effects of Chemically Functionalized and/or Photo-Heated Nanoparticles. Materials 2019, 12, 1078. https://doi.org/10.3390/ma12071078
Pezzi L, Pane A, Annesi F, Losso MA, Guglielmelli A, Umeton C, De Sio L. Antimicrobial Effects of Chemically Functionalized and/or Photo-Heated Nanoparticles. Materials. 2019; 12(7):1078. https://doi.org/10.3390/ma12071078
Chicago/Turabian StylePezzi, Luigia, Alfredo Pane, Ferdinanda Annesi, Maria Adele Losso, Alexa Guglielmelli, Cesare Umeton, and Luciano De Sio. 2019. "Antimicrobial Effects of Chemically Functionalized and/or Photo-Heated Nanoparticles" Materials 12, no. 7: 1078. https://doi.org/10.3390/ma12071078
APA StylePezzi, L., Pane, A., Annesi, F., Losso, M. A., Guglielmelli, A., Umeton, C., & De Sio, L. (2019). Antimicrobial Effects of Chemically Functionalized and/or Photo-Heated Nanoparticles. Materials, 12(7), 1078. https://doi.org/10.3390/ma12071078