Sorption Behaviors of Light Lanthanides(III) (La(III), Ce(III), Pr(III), Nd(III)) and Cr(III) Using Nitrolite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Experimental Methods
3. Results and Discussion
3.1. Effects of Contact Time and pH Value
3.2. Kinetic Models
3.3. Sorption Isotherm
3.4. Thermodynamic Parameters
3.5. Desorption of Metal Ions
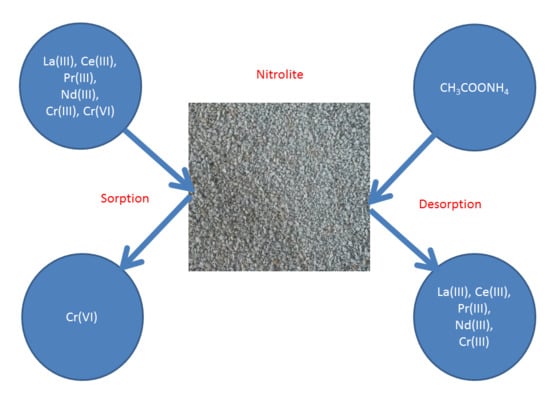
3.6. Removal of Light Lanthanides(III) and Chromium(III) from Chromium(VI) Ions Solutions
3.7. ATR-FTIR Spectra
3.8. Comparison with the Other Natural Sorbents
4. Conclusions
Funding
Conflicts of Interest
References
- Sahan, M.; Kucuker, M.A.; Demirel, B.; Kuchta, K.; Hursthouse, A. Determination of Metal Content of Waste Mobile Phones and Estimation of Their Recovery Potential in Turkey. Int. J. Environ. Res. Public Health 2019, 16, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rager, J.E.; Suh, M.; Chappell, G.A.; Thompson, C.M.; Proctor, D.M. Review of transcriptomic responses to hexavalent chromium exposure in lung cells supports a role of epigenetic mediators in carcinogenesis. Toxicol. Lett. 2019, 305, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ouabo, R.E.; Ogundiran, M.B.; Sangodoyin, A.Y.; Babalola, B.A. Ecological Risk and Human Health Implications of Heavy Metals Contamination of Surface Soil in E-Waste Recycling Sites in Douala, Cameroun. J. Health Pollut. 2019, 9, 190310. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Mendoza-López, M.R. Microbial Bioleaching of Ag, Au and Cu from Printed Circuit Boards of Mobile Phones. Curr. Microbiol. 2019, 76, 536–544. [Google Scholar] [CrossRef]
- Favot, M.; Massarutto, A. Rare-earth elements in the circular economy: The case of yttrium. J. Environ. Manag. 2019, 240, 504–510. [Google Scholar] [CrossRef]
- Larsson, K.; Ekberg, C.; Ødegaard-Jensen, A. Using Cyanex 923 for selective extraction in a high concentration chloride medium on nickel metal hydride battery waste: Part II: Mixer-settler experiments. Hydrometallurgy 2013, 133, 168–175. [Google Scholar] [CrossRef]
- Gupta, B.; Malik, P.; Deep, A. Solvent extraction and separation of tervalent Lanthanides and Yttrium using Cyanex 923. Solvent Extr. Ion Exch. 2003, 21, 239–258. [Google Scholar] [CrossRef]
- Helaly, O.S.; El-Ghany, M.S.A.; Moustafa, M.I.; Abuzaid, A.H.; El-Monem, N.M.A.; Ismail, I.M. Extraction of cerium(IV) using tributyl phosphate impregnated resin from nitric acid medium. Trans. Nonferr. Met. Soc. China 2011, 6326, 1–5. [Google Scholar] [CrossRef]
- Cheremisina, O.V.; Sergeev, V.V.; Chirkst, D.E.; Litvinova, T.E. Thermodynamic investigation into extraction of cerium(III) by tributyl phosphate from phosphoric acid solutions. Russ. J. Non Ferr. Met. 2015, 56, 615–621. [Google Scholar] [CrossRef]
- Bezzina, J.P.; Ogden, M.D.; Moon, E.M.; Soldenhoff, K.L. REE behavior and sorption on weak acid resins from buffered media. J. Ind. Eng. Chem. 2018, 59, 440–455. [Google Scholar] [CrossRef]
- Oh, J.K.; Lee, H.Y.; Kim, S.G. Solvent extraction of chromium from dilute aqueous solution. Geosyst. Eng. 2000, 3, 49–52. [Google Scholar] [CrossRef]
- Lach, J.; Okoniewska, E.; Neczaj, E.; Kacprzak, M. The adsorption of Cr(III) and Cr(VI) on activated carbons in the presence of phenol. Desalination 2008, 223, 249–255. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. Removal of Cr(VI) from aqueous solution by two Lewatit-anion exchange resins. J. Hazard. Mater. 2005, 119, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.; Kaneco, S.; Ohta, K.; Mizuno, T.; Kani, K. Use of some natural and waste materials for waste water treatment. Water Res. 2001, 35, 3738–3742. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ahsan, S.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K. Wastewater treatment with multilayer media of waste and natural indigenous materials. J. Environ. Manag. 2005, 74, 107–110. [Google Scholar] [CrossRef]
- Jachuła, J.; Kołodyńska, D.; Hubicki, Z. Sorption of Cu(II) and Ni(II) ions in the presence of the methylglycinediacetic acid by microporous ion exchangers and sorbents from aqueous solutions. Cent. Eur. J. Chem. 2011, 9, 52–65. [Google Scholar] [CrossRef]
- Ghasemi, N.; Tamri, P.; Khademi, A.; Nezhad, N.S.; Alwi, S.R.W. Linearized Equations of Pseudo Second-order Kinetic for the Adsorption of Pb(II) on Pistacia Atlantica Shells. IERI Procedia 2013, 5, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Über Die Absorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Dubinin, M.M.; Zaverina, E.D.; Radushkevich, L.V. Sorption and structure of active carbons. I. Adsorption of organic vapors. Zh. Fiz. Khim. 1947, 21, 1351–1362. [Google Scholar]
- Gładysz-Płaska, A.; Majdan, M.; Kowalska-Ternes, M. Adsorpcja jonów Nd 3+ na klinoptylolicie. Przem. Chem. 2003, 11, 1435–1439. [Google Scholar]
- Mozgawa, W.; Bajda, T. Spectroscopic study of heavy metals sorption on clinoptilolite. Phys. Chem. Miner. 2005, 31, 706–713. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Q.; Wu, H. Synthesis of Zeolite-like Material by Hydrothermal and Fusion Methods Using Municipal Solid Waste Fly Ash. Procedia Environ. Sci. 2016, 31, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Gougazeh, M.; Buhl, J.C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Asadollahi, M.; Bastani, D.; Kazemian, H. Permeation of single gases through TEG liquid membranes modified by Na-Y nano-zeolite particles. Sep. Purif. Technol. 2010, 76, 120–125. [Google Scholar] [CrossRef]
- Pechar, F.; Rykl, D. Infrared spectra of natural zeolites of the stilbite group. Chem. Zvesti 1981, 35, 189–202. [Google Scholar]
- Aloulou, H.; Bouhamed, H.; Ghorbel, A.; Ben Amar, R.; Khemakhem, S. Elaboration and characterization of ceramic microfiltration membranes from natural zeolite: Application to the treatment of cuttlefish effluents. Desalin. Water Treat. 2018, 95, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Stashkiv, O.; Vasylechko, V.; Gryshchouk, G.; Patsay, I. Solid Phase Extraction of Trace Amounts of Praseodymium Using Transcarpathian Clinoptilolite. Coll. Interfaces 2019, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Kosobucki, P.; Kruk, M.; Buszewski, B. Immobilization of selected heavy metals in sewage sludge by natural zeolites. Bioresour. Technol. 2008, 99, 5972–5976. [Google Scholar] [CrossRef]
- Aja, S.U. Sorption of the rare earth element, Nd, onto kaolinite at 25 °C. Clays Clay Miner. 1998, 46, 103–109. [Google Scholar] [CrossRef]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Chi, R.; Feng, J.; Ding, Y.; Liu, Q. Preparation of modified montmorillonite and its application to rare earth adsorption. Minerals 2019, 9, 747. [Google Scholar] [CrossRef] [Green Version]





| Composition | (K,Na,1/2Ca)2 ·Al2O3·10SiO2·H2O |
|---|---|
| Total Capacity | 0.7 eq L−1 (NH4+ ions) |
| Bead size | 0.63–1.4 mm |
| Max temp. range | 1023 K |
| Name | Linear Form |
|---|---|
| Pseudo-first-order (PFO) | |
| Pseudo-second-order 1 (PSO1) | |
| Pseudo-second-order 2 (PSO2) | |
| Pseudo-second-order 3 (PSO3) | |
| Pseudo-second-order 4 (PSO4) | |
| Elovich | |
| Intra-particle diffusion |
| PFO | PSO1 | ||||||
| Metal Ions | pH | k1 (1/min) | qe (mg/g) | R2 | k2 (g/mg·min) | qe (mg/g) | R2 |
| La(III) | 2 | 0.0131 | 1.3733 | 0.9114 | 0.0537 | 2.3144 | 0.9989 |
| La(III) | 5 | 0.0154 | 2.2280 | 0.8485 | 0.0535 | 3.7597 | 0.9996 |
| Ce(III) | 2 | 0.0141 | 0.6544 | 0.8727 | 0.0790 | 2.0347 | 0.9996 |
| Ce(III) | 5 | 0.0138 | 1.5482 | 0.8080 | 0.0617 | 3.4466 | 0.9997 |
| Pr(III) | 2 | 0.0161 | 0.3756 | 0.8658 | 0.1120 | 1.7224 | 0.9997 |
| Pr(III) | 5 | 0.0130 | 1.4055 | 0.7887 | 0.0609 | 3.1213 | 0.9996 |
| Nd(III) | 2 | 0.0145 | 0.6742 | 0.8983 | 0.0720 | 1.7481 | 0.9993 |
| Nd(III) | 5 | 0.0105 | 1.4310 | 0.7559 | 0.0604 | 2.9862 | 0.9997 |
| Cr(III) | 2 | 0.0093 | 0.0669 | 0.8514 | 0.2048 | 0.6031 | 0.9957 |
| Cr(III) | 5 | 0.00935 | 3.0528 | 0.9156 | 0.01665 | 2.46105 | 0.99626 |
| Elovich | Intra-particle Diffusion | ||||||
| pH | α (g/mg·min) | β (mg/g) | R2 | k (mg/g·min0.5) | C (mg/g) | R2 | |
| La(III) | 2 | 1.1954 | 2.9111 | 0.9663 | 0.1095 | 0.5746 | 0.7815 |
| La(III) | 5 | 3.4280 | 1.9769 | 0.9525 | 0.1686 | 1.1647 | 0.6784 |
| Ce(III) | 2 | 1.1061 | 3.1860 | 0.9286 | 0.0969 | 0.5372 | 0.7325 |
| Ce(III) | 5 | 2.5139 | 2.0221 | 0.9273 | 0.1584 | 1.0301 | 0.6545 |
| Pr(III) | 2 | 1.1945 | 3.9270 | 0.9375 | 0.0806 | 0.4867 | 0.7422 |
| Pr(III) | 5 | 2.1554 | 2.2328 | 0.9480 | 0.1450 | 0.8882 | 0.6875 |
| Nd(III) | 2 | 0.7362 | 3.5966 | 0.9504 | 0.0867 | 0.3825 | 0.7978 |
| Nd(III) | 5 | 1.8693 | 2.2813 | 0.9405 | 0.1399 | 0.8297 | 0.6811 |
| Cr(III) | 2 | 0.8688 | 13.4576 | 0.9646 | 0.0259 | 0.1900 | 0.7247 |
| Cr(III) | 5 | 0.4297 | 2.48194 | 0.9687 | 0.12051 | 0.2753 | 0.89843 |
| Metal Ions | Langmuir | Freundlich | Dubinin–Radushkevich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | b | RL | R2 | Kf | n | R2 | β | qm | E | R2 | |
| La(III) | 4.77 | 0.0698 | 0.0278 | 0.9990 | 1.1952 | 4.49 | 0.9066 | 1.96 × 10−9 | 0.000246 | 15.99 | 0.9575 |
| Ce(III) | 4.45 | 0.0603 | 0.0321 | 0.9982 | 1.1849 | 4.73 | 0.9027 | 2.03 × 10−9 | 0.000232 | 15.71 | 0.9703 |
| Pr(III) | 4.30 | 0.0478 | 0.0401 | 0.9975 | 1.1254 | 4.76 | 0.9439 | 2.05 × 10−9 | 0.000223 | 15.63 | 0.9776 |
| Nd(III) | 4.13 | 0.0438 | 0.0437 | 0.9974 | 1.1042 | 4.88 | 0.9559 | 2.08 × 10−9 | 0.000212 | 15.49 | 0.9882 |
| Cr(III) | 2.39 | 0.0768 | 0.0254 | 0.9989 | 1.0487 | 8.05 | 0.9647 | 2.26 × 10−9 | 0.000289 | 14.87 | 0.9485 |
| Metal Ions | ΔH° (kJ/mol) | ΔS° (kJ/K·mol) | ΔG° (kJ/mol) | ||
|---|---|---|---|---|---|
| 293 K | 303 K | 323 K | |||
| La(III) | 10.224 | 0.0154 | 5.67 | 5.52 | 5.04 |
| Ce(III) | 10.867 | 0.0154 | 6.30 | 6.18 | 5.67 |
| Pr(III) | 13.305 | 0.0200 | 7.39 | 7.16 | 6.58 |
| Nd(III) | 11.217 | 0.0128 | 7.57 | 6.94 | 7.09 |
| Cr(III) | 17.580 | 0.0264 | 9.72 | 9.63 | 8.62 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, G. Sorption Behaviors of Light Lanthanides(III) (La(III), Ce(III), Pr(III), Nd(III)) and Cr(III) Using Nitrolite. Materials 2020, 13, 2256. https://doi.org/10.3390/ma13102256
Wójcik G. Sorption Behaviors of Light Lanthanides(III) (La(III), Ce(III), Pr(III), Nd(III)) and Cr(III) Using Nitrolite. Materials. 2020; 13(10):2256. https://doi.org/10.3390/ma13102256
Chicago/Turabian StyleWójcik, Grzegorz. 2020. "Sorption Behaviors of Light Lanthanides(III) (La(III), Ce(III), Pr(III), Nd(III)) and Cr(III) Using Nitrolite" Materials 13, no. 10: 2256. https://doi.org/10.3390/ma13102256
APA StyleWójcik, G. (2020). Sorption Behaviors of Light Lanthanides(III) (La(III), Ce(III), Pr(III), Nd(III)) and Cr(III) Using Nitrolite. Materials, 13(10), 2256. https://doi.org/10.3390/ma13102256