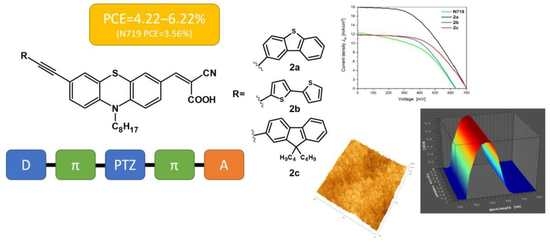
Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Electrochemical Properties
3.3. Structure Optimization and Frontier Molecular Orbitals
3.4. Experimental and Theoretical Optical Properties
3.5. Evaluation of Phenothiazine Derivatives as Sensitizers in DSSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Yin, J.-F.; Velayudham, M.; Bhattacharya, D.; Lin, H.-C.; Lu, K.-L. Structure optimization of ruthenium photosensitizers for efficient dye-sensitized solar cells—A goal toward a “bright” future. Coord. Chem. Rev. 2012, 256, 3008–3035. [Google Scholar] [CrossRef]
- Rout, Y.; Gautam, P.; Misra, R. Unsymmetrical and Symmetrical Push–Pull Phenothiazines. J. Org. Chem. 2017, 82, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yella, A.; Mai, C.-L.; Zakeeruddin, S.-M.; Chang, S.-N.; Hsieh, C.-H.; Yeh, C.-Y.; Grätzel, M. Molecular Engineering of Push–Pull Porphyrin Dyes for Highly Efficient Dye-Sensitized Solar Cells: The Role of Benzene Spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Duvva, N.; Prasanthkumara, S.; Giribabu, L. Influence of strong electron donating nature of phenothiazine on A3B-type porphyrin based dye sensitized solar cells. Sol. Energy 2019, 184, 620–627. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Li, Y. Phenothiazine organic dyes containing dithieno[3,2-b:2′,3′-d]pyrrole (DTP) units for dye-sensitized solar cells. Sol. Energy 2017, 157, 94–102. [Google Scholar] [CrossRef]
- Al-Busaidi, J.I.; Haque, A.; Rasbi, N.K.A.; Khan, M.S. Phenothiazine-based derivatives for optoelectronic applications: A review. Synthetic Met. 2019, 257, 116189–116205. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, M.; Wu, H.; Yang, L.; Li, R.; Wang, P. Donor/Acceptor Indenoperylene Dye for Highly Efficient Organic Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 3799–3802. [Google Scholar] [CrossRef]
- Sakong, C.; Kim, H.J.; Kim, S.H.; Namgoong, J.W.; Park, J.H.; Ryu, J.-H.; Kim, B.; Ko, M.J.; Kim, J.P. Synthesis and applications of new triphenylamine dyes with donor-donor-(bridge)-acceptor structure for organic dye-sensitized solar cells. New J. Chem. 2012, 36, 2025–2032. [Google Scholar] [CrossRef]
- Zych, D.; Kurpanik, A.; Slodek, A.; Maron, A.; Pajak, M.; Szafraniec-Gorol, G.; Matussek, M.; Krompiec, S.; Schab-Balcerzak, E.; Kotowicz, S.; et al. NCN-Coordinating Ligands based on Pyrene Structure with Potential Application in Organic Electronics. Chem. Eur. J. 2017, 23, 15746–15758. [Google Scholar] [CrossRef] [PubMed]
- Zych, D.; Slodek, A.; Matussek, M.; Filapek, M.; Szafraniec-Gorol, G.; Maslanka, S.; Krompiec, S.; Kotowicz, S.; Schab-Balcerzak, E.; Smolarek, K.; et al. 4′-Phenyl-2,2′:6′,2″-terpyridine derivatives-synthesis, potential application and the influence of acetylene linker on their properties. Dyes Pigments 2017, 146, 331–343. [Google Scholar] [CrossRef]
- Hua, Y.; Chang, S.; Wang, H.; Huang, D.; Zhao, J.; Chen, T.; Wong, W.-Y.; Wong, W.-K.; Zhu, X. New phenothiazine-based dyes for efficient dye-sensitized solar cells: Positioning effect of a donor group on the cell performance. J. Power Sources 2013, 243, 253–259. [Google Scholar] [CrossRef]
- Czaplinska, B.; Maron, A.; Malecki, J.G.; Szafraniec-Gorol, G.; Matussek, M.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Danikiewicz, W.; Musiol, R.; Slodek, A. Comprehensive exploration of the optical and biological properties of new quinoline based cellular probes. Dyes Pigments 2017, 144, 119–132. [Google Scholar] [CrossRef]
- Chai, Q.; Li, W.; Wu, Y.; Pei, K.; Liu, J.; Geng, Z.; Tian, H.; Zhu, W. Effect of a long alkyl group on cyclopentadithiophene as a conjugated bridge for D-A-π-A organic sensitizers: IPCE, electron diffusion Length, and charge recombination. ACS Appl. Mater. Interfaces 2014, 6, 14621–14630. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Golba, S.; Zimosz, S.; Gnida, P.; Schab-Balcerzak, E. Dyes based on the D/A-acetylene linker-phenothiazine system for developing efficient dye-sensitized solar cells. J. Mater. Chem. C 2019, 7, 5830–5840. [Google Scholar] [CrossRef]
- Szafraniec-Gorol, G.; Slodek, A.; Filapek, M.; Boharewicz, B.; Iwan, A.; Jaworska, M.; Zur, L.; Soltys, M.; Pisarska, J.; Grudzka-Flak, I.; et al. Novel iridium (III) complexes based on 2-(2, 2′-bithien-5-yl)-quinoline. Synthesis, photophysical, photochemical and DFT studies. Mat. Chem. Phys. 2015, 162, 498–508. [Google Scholar] [CrossRef]
- Zych, D.; Slodek, A. Sensitizers for DSSC containing triazole motif with acceptor/donor substituents-Correlation between theoretical and experimental data in prediction of consistent photophysical parameters. J. Mol. Struct. 2020, 1207, 127771–127776. [Google Scholar] [CrossRef]
- Soliman, H.N.; Yahia, I.S. Synthesis and technical analysis of 6-butyl-3-[(4-chlorophenyl)diazenyl]-4-hydroxy-2H-pyrano[3,2-c] quinoline-2,5(6H)-dione as a new organic semiconductor: Structural, optical and electronic properties. Dyes Pigments 2020, 176, 108199–108205. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Maron, A.; Golba, S.; Schab-Balcerzak, E.; Janeczek, H.; Siwy, M.; Mackowski, S. Fluorene vs carbazole substituent at quinoline core toward organic electronics. Dyes Pigments 2019, 166, 98–106. [Google Scholar] [CrossRef]
- Mao, M.; Wang, J.-B.; Liu, X.-L.; Wu, G.-H.; Fang, X.-Q.; Song, Q.-H. Insight into the effects of modifying chromophores on the performance of quinoline-based dye-sensitized solar cells. Spectrochim. Acta A 2018, 190, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Slodek, A.; Zych, D.; Maron, A.; Gawecki, R.; Mrozek-Wilczkiewicz, A.; Malarz, K.; Musioł, R. Phenothiazine derivatives–synthesis, characterization, and theoretical studies with an emphasis on the solvatochromic properties. J. Mol. Liq. 2019, 285, 515–525. [Google Scholar] [CrossRef]
- Ramasamy, S.; Boopathy, M.; Johnsanthoshkumar, S.; Subramanian, K. Structural engineering of poly-(methacrylate) bearing push-pull type pendants oxindole-phenothiazine with tetrazole anchoring acceptor for efficient organic photovoltaic cells. Polymer 2017, 115, 128–136. [Google Scholar] [CrossRef]
- Buene, A.F.; Uggerud, N.; Economopoulos, S.P.; Gautun, O.R.; Hoff, B.H. Effect of π-linkers on phenothiazine sensitizers for dye-sensitized solar cells. Dyes Pigments 2018, 151, 263–271. [Google Scholar] [CrossRef]
- Zhu, B.-Y.; Wu, L.; Ye, Q.; Gao, J.-R.; Han, L. Asymmetric double donor-π-acceptor dyes based on phenothiazine and carbazole donors for dye-sensitized solar cells. Tetrahedron 2017, 73, 6307–6315. [Google Scholar] [CrossRef]
- Eiamprasert, U.; Sudchanham, J.; Surawatanawong, P.; Pakawatpanurut, P.; Kiatisevi, S. Additional donor bridge as a design approach for multi-anchoring dyes for highly efficient dye-sensitized solar cells. J. Photochem. Photobio. A 2018, 352, 86–97. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, Q.; Wu, W.; Pei, H.; Liu, B.; Tian, H. Starburst triarylamine based dyes for efficient dye-sensitized solar cells. J. Org. Chem. 2008, 73, 3791–3797. [Google Scholar] [CrossRef]
- Tang, J.; Hua, J.; Wu, W.; Li, J.; Jin, Z.; Long, Y.; Tian, H. New starburst sensitizer with carbazole antennas for efficient and stable dye-sensitized solar cells. Energy Environ. Sci. 2010, 3, 1736–1745. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Hsu, Y.-C.; Lin, J.T.; Chen, H.-C.; Hsu, C.-P. Organic dyes containing 1H-phenanthro[9,10-d]imidazole conjugation for solar cells. J. Phys. Chem. C 2007, 111, 18785–18793. [Google Scholar] [CrossRef]
- Kraemer, C.S.; Zeitler, K.; Mueller, T.J.J. Synthesis of Functionalized Ethynylphenothiazine Fluorophores. Org. Lett. 2000, 2, 3723–3726. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lu, R.; Zhou, H.; Zhang, X.; Xu, T.; Liu, X.; Zhao, Y. Synthesis of linear monodisperse vinylene-linked phenothiazine oligomers. Tetrahedron Lett. 2007, 48, 7582–7585. [Google Scholar] [CrossRef]
- Slodek, A.; Filapek, M.; Szafraniec, G.; Grudzka, I.; Pisarski, W.A.; Malecki, J.G.; Zur, L.; Grela, M.; Danikiewicz, W.; Krompiec, S. Synthesis, Electrochemistry, Crystal Structures and Optical Properties of Novel Quinoline Derivatives with 2,2′-Bithiophene Motif. Eur. J. Org. Chem. 2014, 24, 5256–5264. [Google Scholar] [CrossRef]
- Slodek, A.; Matussek, M.; Filapek, M.; Szafraniec-Gorol, G.; Szlapa, A.; Grudzka-Flak, I.; Szczurek, M.; Malecki, J.G.; Maron, A.; Schab-Balcerzak, E.; et al. Small donor-acceptor molecules based on novel quinoline-fluorene system with promising photovoltaic properties. Eur. J. Org. Chem. 2016, 14, 2500–2508. [Google Scholar] [CrossRef]
- Duddu, S.P.; Grant, D.J.W. The use of thermal analysis in the assessment of crystal disruption. Thermochim. Acta 1995, 248, 131–145. [Google Scholar] [CrossRef]
- Kula, S.; Szlapa-Kula, A.; Fabianczyk, A.; Gnida, P.; Libera, M.; Bujak, K.; Siwy, M.; Schab-Balcerzak, E. Effect of thienyl units in cyanoacrylic acid derivatives toward dye-sensitized solar cells. J. Photochem. Photobiol. B 2019, 197, 111555. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Pereira, A.; Ivanou, D.; Mendes, A.; Raposo, M.M. Benzothiadiazole derivatives functionalized with two different (hetero)aromatic donor groups: Synthesis and evaluation as TiO2 sensitizers for DSSCs. Dyes Pigments 2018, 151, 89–94. [Google Scholar] [CrossRef]
- Data, P.; Zassowski, P.; Lapkowski, M.; Grazulevicius, J.V.; Kukhta, N.A.; Reghu, R.R. Electrochromic behavior of triazine based ambipolar compounds. Electrochim. Acta 2016, 192, 283–295. [Google Scholar] [CrossRef]
- Kathiravan, A.; Panneerselvam, M.; Sundaravel, K.; Pavithra, N.; Srinivasan, V.; Jaccob, S.M. Unravelling the effect of anchoring groups on the ground and excited-state properties of pyrene using computational and spectroscopic methods. Phys. Chem. Chem. Phys. 2016, 18, 13332–13345. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaussian, Version 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]












| Code | Eoxonset (V) | Eredonset (V) | HOMO (eV) | LUMO (eV) | EgCV (eV) | EgDPV (eV) |
|---|---|---|---|---|---|---|
| 2a | 0.40 | −1.32 | −5.50 | −3.78 | 1.72 | 1.76 |
| 2b | 0.42 | −1.34 | −5.52 | −3.76 | 1.76 | 1.68 |
| 2c | 0.47 | −1.22 | −5.57 | −3.88 | 1.69 | 1.68 |
| IP = −5.1 − Eoxonset; EA = −5.1 − Eredonset; EgCV = Eoxonset − Eredonset | ||||||
| Code | HOMO-1 | HOMO | LUMO | LUMO+1 |
|---|---|---|---|---|
| 2a |  |  |  |  |
| −5.95 eV | −5.39 eV | −2.60 eV | −1.50 eV | |
| 2b |  |  |  |  |
| −5.80 eV | −5.23 eV | −2.60 eV | −1.94 eV | |
| 2c |  |  |  |  |
| −5.82 eV | −5.28 eV | −2.52 eV | −1.60 eV |
| Code | HOMO (eV) | LUMO (eV) | ΔE (eV) |
|---|---|---|---|
| 2a/(TiO2)9 |  |  | 1.95 |
| −5.35 | −3.40 | ||
| 2b/(TiO2)9 |  |  | 1.80 |
| −5.19 | −3.39 | ||
| 2c/(TiO2)9 |  |  | 1.89 |
| −5.28 | −3.39 |
| Code | λmax (nm) (ε (104M−1cm−1)) | EgOPT (eV) |
|---|---|---|
| 1a | 249 (8.4), 268 (9.0), 290 (9.0), 395 (1.6) | 2.63 |
| 1b | 279 (5.8), 357 (2.6), 399 (2.0) | 2.61 |
| 1c | 296 (5.1), 307 (5.3), 325 (5.7), 395 (2.0) | 2.63 |
| 2a | 248 (2.6), 265 (2.3), 313 (2.6), 440 (0.87) | 2.30 |
| 2b | 325 (2.4), 453 (1.4) | 2.25 |
| 2c | 284 (2.1), 325 (2.4), 424 (0.63) | 2.24 |
| EgOPT = 1240/λ | ||
| Code | Exp (nm) | Exchange-Correlation Functional | Calculated Wavelengths (nm) (Oscillator Strengths) | Transitions | LHE |
|---|---|---|---|---|---|
| 2a | 440 | CAM-B3LYP | 371.80 (0.7598) | H-1->LUMO (19%), HOMO->LUMO (65%) | 0.826 |
| wB97XD | 355.93 (0.8533) | HOMO->LUMO (55%), H-1->LUMO (21%) | 0.860 | ||
| 2b | 452 | CAM-B3LYP | 378.12 (1.3308) | HOMO->LUMO (46%), H-1->LUMO (23%), HOMO->L+1 (19%) | 0.953 |
| wB97XD | 362.45 (1.5094) | HOMO->LUMO (35%), HOMO->L+1 (28%), H-1->LUMO (21%) | 0.969 | ||
| 2c | 423 | CAM-B3LYP | 383.67 (0.9218) | HOMO->LUMO (65%), H-1->LUMO (22%) | 0.880 |
| wB97XD | 373.24 (0.9951) | HOMO->LUMO (57%), H-1->LUMO (25%) | 0.899 |
| Sensitizer | Voc (mV) | Jsc (mA/cm2) | FF (−) | PCE (%) |
|---|---|---|---|---|
| N719 | 631 | 12.34 | 0.46 | 3.56 |
| 2a | 700 | 17.96 | 0.48 | 6.22 |
| 2b | 631 | 11.87 | 0.54 | 4.22 |
| 2c | 703 | 12.08 | 0.56 | 4.80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slodek, A.; Zych, D.; Szafraniec-Gorol, G.; Gnida, P.; Vasylieva, M.; Schab-Balcerzak, E. Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight. Materials 2020, 13, 2292. https://doi.org/10.3390/ma13102292
Slodek A, Zych D, Szafraniec-Gorol G, Gnida P, Vasylieva M, Schab-Balcerzak E. Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight. Materials. 2020; 13(10):2292. https://doi.org/10.3390/ma13102292
Chicago/Turabian StyleSlodek, Aneta, Dawid Zych, Grażyna Szafraniec-Gorol, Paweł Gnida, Marharyta Vasylieva, and Ewa Schab-Balcerzak. 2020. "Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight" Materials 13, no. 10: 2292. https://doi.org/10.3390/ma13102292
APA StyleSlodek, A., Zych, D., Szafraniec-Gorol, G., Gnida, P., Vasylieva, M., & Schab-Balcerzak, E. (2020). Investigations of New Phenothiazine-Based Compounds for Dye-Sensitized Solar Cells with Theoretical Insight. Materials, 13(10), 2292. https://doi.org/10.3390/ma13102292