Thermal Analysis of High Entropy Rare Earth Oxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Synthesis
2.2. Laser Melting and Splat Quenching
2.3. Microprobe Analysis
2.4. Room Temperature X-ray Diffraction
2.5. High Temperature X-ray Diffraction
2.6. Differential Thermal Analysis
2.7. Calphad Modeling
3. Results
3.1. Chemical Composition
3.2. Phases after Solution Combustion Synthesis and Annealing
3.3. Phases after Laser Melting, Splat Quenching, and Annealing
3.4. Temperatures and Enthalpies of Phase Transformations from DTA Experiments
3.5. Volume Changes and Thermal Expansion from High-Temperature XRD
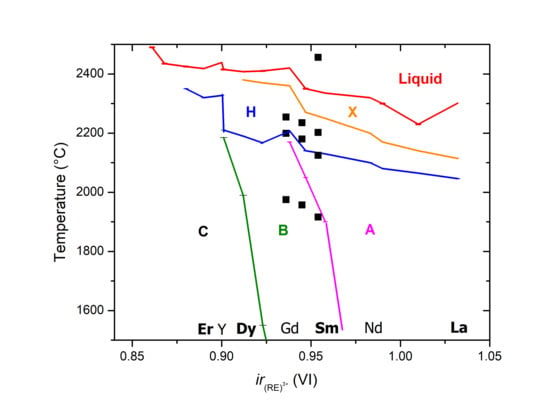
4. Discussion
4.1. Experiment vs. Calphad Predictions
4.1.1. C-B Transition
4.1.2. B-A-H-X Transitions
4.1.3. Biphasic Fields
4.2. Thermal Expansion and Volume Change on Mixing
4.2.1. Volume Change on C-B Transition
4.2.2. Thermal Expansion of B-Type Solid Solutions
4.2.3. Volume Changes on B-A and H-X Transformations
4.2.4. Volumes of H-Type Solid Solution vs. Pure Oxides
4.3. Increase of Melting Temperature in HE-RE
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Castle, E.; Csanadi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and Properties of High-Entropy Ultra-High Temperature Carbides. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E.; Zhou, Y. Synthesis of single-phase high-entropy carbide powders. Scr. Mater. 2019, 162, 90–93. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef]
- Tallarita, G.; Licheri, R.; Garroni, S.; Orrù, R.; Cao, G. Novel processing route for the fabrication of bulk high-entropy metal diborides. Scr. Mater. 2019, 158, 100–104. [Google Scholar] [CrossRef]
- Liu, D.; Wen, T.; Ye, B.; Chu, Y. Synthesis of superfine high-entropy metal diboride powders. Scr. Mater. 2019, 167, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Rák, Z.; Maria, J.P.; Brenner, D.W. Evidence for Jahn-Teller compression in the (Mg, Co, Ni, Cu, Zn)O entropy-stabilized oxide: A DFT study. Mater. Lett. 2018, 217, 300–303. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [Green Version]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Pei, X.; Tang, L.; Cheng, H.; Li, Z.; Li, C.; Zhang, X.; An, L. A five-component entropy-stabilized fluorite oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Jiang, S.; Hu, T.; Gild, J.; Zhou, N.; Nie, J.; Qin, M.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B.; Sarkar, A.; et al. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, 1806236. [Google Scholar] [CrossRef]
- Breitung, B.; Wang, Q.; Schiele, A.; Tripkovic, D.; Sarkar, A.; Velasco, L.; Wang, D.; Bhattacharya, S.S.; Hahn, H.; Brezesinski, T. Gassing Behavior of High-Entropy Oxide Anode and Oxyfluoride Cathode Probed Using Differential Electrochemical Mass Spectrometry. Batter. Supercaps 2020, 3, 361–369. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Li, Z.; Lu, Y.; Velasco, L.; Bhattacharya, S.S.; Brezesinski, T.; Hahn, H.; Breitung, B. High entropy oxides as anode material for Li-ion battery applications: A practical approach. Electrochem. Commun. 2019, 100, 121–125. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; de Biasi, L.; Kuebel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H.; et al. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hume-Rothery, W.; Powell, H.M. The theory of superlattice structure in alloys. Z. Krist. 1935, 91, 23–47. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Laws of crystal chemistry. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Charykova, M.V.; Krivovichev, S.V. The concept of mineral systems and its application to the study of mineral diversity and evolution. Eur. J. Mineral. 2018, 30, 219–230. [Google Scholar] [CrossRef]
- Tomilin, I.A.; Kaloshkin, S.D. High entropy alloys—Semi-impossible regular solid solutions? Mater. Sci. Technol. 2015, 31, 1231–1234. [Google Scholar] [CrossRef]
- Goldschmidt, V.M.; Ulrich, F.; Barth, T. Geochemical distribution laws of the elements. IV. The crystal structure of the oxides of the rare earth metals. In Skrifter Utgit av det Norske Videnskap-Akademi i Oslo. (I) Matem.-Naturvid. Klasse; Facsimile Publisher: London, UK, 1925; pp. 5–24. [Google Scholar]
- Kennedy, B.J.; Avdeev, M. The structure of B-type Sm2O3. A powder neutron diffraction study using enriched 154Sm. Solid State Sci. 2011, 13, 1701–1703. [Google Scholar] [CrossRef]
- Glushkova, V.B.; Boganov, A.G. Polymorphism of rare-earth sesquioxides. Bull. Acad. Sci. USSR Div. Chem. Sci. 1965, 14, 1101–1107. [Google Scholar] [CrossRef]
- Rustad, J.R. Density functional calculations of the enthalpies of formation of rare-earth orthophosphates. Am. Mineral. 2012, 97, 791–799. [Google Scholar] [CrossRef]
- Guo, B.; Harvey, A.S.; Neil, J.; Kennedy, I.M.; Navrotsky, A.; Risbud, S.H. Atmospheric pressure synthesis of heavy rare earth sesquioxides nanoparticles of the uncommon monoclinic phase. J. Am. Ceram. Soc. 2007, 90, 3683–3686. [Google Scholar] [CrossRef]
- Gutowski, K.E.; Bridges, N.J.; Rogers, R.D. Actinide Structural Chemistry. In The Chemistry of the Actinide and Transactinide Elements; Morss, L.R., Edelstein, N.M., Fuger, J., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Foex, M.; Pierre Traverse, J. Polymorphism of rare earth sesquioxides at high temperatures. Bull. Soc. Fr. Miner. Cristal. 1966, 89, 184–205. [Google Scholar]
- Foex, M.; Traverse, J.P. Investigations about crystalline transformation in rare earths sesquioxides at high temperatures. Rev. Int. Hautes Temp. Refract. 1966, 3, 429–453. [Google Scholar]
- Aldebert, P.; Traverse, J.P. Neutron diffraction study of the high temperature structures of lanthanum oxide and neodymium oxide. Mater. Res. Bull. 1979, 14, 303–323. [Google Scholar] [CrossRef]
- Pavlik, A.; Ushakov, S.V.; Navrotsky, A.; Benmore, C.J.; Weber, R.J.K. Structure and thermal expansion of Lu2O3 and Yb2O3 up to the melting points. J. Nucl. Mater. 2017, 495, 385–391. [Google Scholar] [CrossRef]
- Coutures, J.; Rouanet, A.; Verges, R.; Foex, M. High-temperature study of systems formed by lanthanum sesquioxide and lanthanide sesquioxides. I. Phase diagrams (1400°C < T < T liquid). J. Solid State Chem. 1976, 17, 171–182. [Google Scholar]
- Tracy, C.L.; Lang, M.; Zhang, F.; Trautmatm, C.; Ewing, R.C. Phase transformations in Ln2O3 materials irradiated with swift heavy ions. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 174101. [Google Scholar] [CrossRef] [Green Version]
- Schneider, S.J.; Roth, R.S. Phase equilibriums in system involving the rare earth oxides. II. Solid-state reactions in trivalent rare earth oxide systems. J. Res. Natl. Bur. Stand. Sect. A 1960, 64, 317–332. [Google Scholar] [CrossRef]
- Chudinovych, O.V.; Korichev, S.F.; Andrievskaya, E.R. Interaction of Yttrium, Lanthanum, and Samarium Oxides at 1600 °C. Powder Metall. Met. Ceram. 2020, 58, 599–607. [Google Scholar] [CrossRef]
- Qi, J.; Guo, X.; Mielewczyk-Gryn, A.; Navrotsky, A. Formation enthalpies of LaLn׳O3 (Ln׳=Ho, Er, Tm and Yb) interlanthanide perovskites. J. Solid State Chem. 2015, 227, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Artini, C.; Pani, M.; Lausi, A.; Costa, G.A. Stability of interlanthanide perovskites ABO3 (A≡La-Pr; B≡Y, Ho-Lu). J. Phys. Chem. Solids 2016, 91, 93–100. [Google Scholar] [CrossRef]
- Artini, C. Crystal chemistry, stability and properties of interlanthanide perovskites: A review. J. Eur. Ceram. Soc. 2017, 37, 427–440. [Google Scholar] [CrossRef]
- Pavunny, S.P.; Kumar, A.; Misra, P.; Scott, J.F.; Katiyar, R.S. Properties of the new electronic device material LaGdO3. Phys. Status Solidi B 2014, 251, 131–139. [Google Scholar] [CrossRef]
- Siai, A.; Haro-Gonzalez, P.; Horchani Naifer, K.; Ferid, M. Optical temperature sensing of Er3+/Yb3+ doped LaGdO3 based on fluorescence intensity ratio and lifetime thermometry. Opt. Mater. 2018, 76, 34–41. [Google Scholar] [CrossRef]
- Gutierrez-Cano, V.; Rodriguez, F.; Gonzalez, J.A.; Valiente, R. Upconversion and Optical Nanothermometry in LaGdO3: Er3+ Nanocrystals in the RT to 900 K Range. J. Phys. Chem. C 2019, 123, 29818–29828. [Google Scholar] [CrossRef]
- Fagg, D.P.; Marozau, I.P.; Shaula, A.L.; Kharton, V.V.; Frade, J.R. Oxygen permeability, thermal expansion and mixed conductivity of GdxCe0.8−xPr0.2O2−δ, x=0, 0.15, 0.2. J. Solid State Chem. 2006, 179, 3347–3356. [Google Scholar] [CrossRef]
- Tseng, K.-P.; Yang, Q.; McCormack, S.J.; Kriven, W.M. High-entropy, phase-constrained, lanthanide sesquioxide. J. Am. Ceram. Soc. 2020, 103, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Aldebert, P.; Dianoux, A.J.; Traverse, J.P. Neutron scattering evidence for fast ionic oxygen diffusion in the high temperature phases of lanthanum oxide. J. Phys. 1979, 40, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Erukhimovitch, V.; Mordekoviz, Y.; Hayun, S. Spectroscopic study of ordering in non-stoichiometric magnesium aluminate spinel. Am. Mineral. 2015, 100, 1744. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Shvarev, A.; Alexeev, T.; Kapush, D.; Navrotsky, A. Drop-and-catch (DnC) calorimetry using aerodynamic levitation and laser heating. J. Am. Ceram. Soc. 2017, 100, 754–760. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The genesis of a modern open-source all purpose crystallography software package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Rietveld, H.M. The Rietveld method. Phys. Scr. 2014, 89, 098002. [Google Scholar] [CrossRef]
- Weber, J.K.R.; Tamalonis, A.; Benmore, C.J.; Alderman, O.L.G.; Sendelbach, S.; Hebden, A.; Williamson, M.A. Aerodynamic levitator for in situ x-ray structure measurements on high temperature and molten nuclear fuel materials. Rev. Sci. Instrum. 2016, 87, 073902. [Google Scholar] [CrossRef]
- Guazzoni, G.E. High-Temperature Spectral Emittance of Oxides of Erbium, Samarium, Neodymium and Ytterbium. Appl. Spectrosc. 1972, 26, 60–65. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A.; Weber, R.J.K.; Neuefeind, J.C. Structure and Thermal Expansion of YSZ and La2Zr2O7 Above 1500°C from Neutron Diffraction on Levitated Samples. J. Am. Ceram. Soc. 2015, 98, 3381–3388. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Maram, P.S.; Kapush, D.; Pavlik, A.J., III; Fyhrie, M.; Gallington, L.C.; Benmore, C.J.; Weber, R.; Neuefeind, J.C.; McMurray, J.W.; et al. Phase transformations in oxides above 2000°C: Experimental technique development. Adv. Appl. Ceram. 2018, 117, s82–s89. [Google Scholar] [CrossRef]
- McCormack, S.J.; Tamalonis, A.; Weber, R.J.K.; Kriven, W.M. Temperature gradients for thermophysical and thermochemical property measurements to 3000 °C for an aerodynamically levitated spheroid. Rev. Sci. Instrum. 2019, 90, 015109. [Google Scholar] [CrossRef] [PubMed]
- Toby, B.H. Estimating observed structure factors without a structure. In International Tables for Crystallography; Gilmore, C.J., Kaduk, J.A., Schenk, H., Eds.; International Union of Crystallography Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Boettinger, W.J.; Kattner, U.R.; Moon, K.-W.; Perepezko, J.J. DTA and Heat-flux DSC Measurements of Alloy Melting and Freezing; National Institute of Standards and Technology: Washington, DC, USA, 2006.
- Ushakov, S.V.; Navrotsky, A. Direct measurements of fusion and phase transition enthalpies in lanthanum oxide. J. Mater. Res. 2011, 26, 845–847. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A. Experimental approaches to the thermodynamics of ceramics above 1500 °C. J. Am. Ceram. Soc. 2012, 95, 1463–1482. [Google Scholar] [CrossRef]
- Navrotsky, A.; Ushakov, S.V. Hot matters—Experimental methods for high-temperature property measurement. Am. Ceram. Soc. Bull. 2017, 96, 22–28. [Google Scholar]
- Preston-Thomas, H. The International Temperature Scale of 1990 (ITS-90). Metrologia 1990, 27, 3–10. [Google Scholar] [CrossRef]
- Schneider, S.J. Cooperative determination of the melting point of alumina. Pure Appl. Chem. 1970. [Google Scholar] [CrossRef] [Green Version]
- Hlavac, J. Melting temperatures of refractory oxides. Part I. Pure Appl. Chem. 1982, 54, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, L.; Ågren, J. CALPHAD, first and second generation—Birth of the materials genome. Scr. Mater. 2014, 70, 3–6. [Google Scholar] [CrossRef]
- Lukas, H.L.; Fries, S.G.; Sundman, B. Computational Thermodynamics: The CALPHAD Method; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Sundman, B.; Kattner, U.R.; Palumbo, M.; Fries, S.G. OpenCalphad—A free thermodynamic software. Integr. Mater. Manuf. Innov. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Pelton, A.D.; Kang, Y.-B. Modeling short-range ordering in solutions. Int. J. Mater. Res. 2007, 98, 907–917. [Google Scholar] [CrossRef]
- Zinkevich, M. Thermodynamics of rare earth sesquioxides. Prog. Mater. Sci. 2007, 52, 597–647. [Google Scholar] [CrossRef]
- Zhang, Y.; Jung, I.-H. Critical evaluation of thermodynamic properties of rare earth sesquioxides (RE = La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Sc and Y). Calphad Comput. Coupling Phase Diagr. 2017, 58, 169–203. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Beneš, O.; Kovács, A.; Manara, D.; Sedmidubský, D.; Gorokhov, L.; Iorish, V.S.; Yungman, V.; Shenyavskaya, E.; Osina, E. The Thermodynamic Properties of the f-Elements and their Compounds. Part 2. The Lanthanide and Actinide Oxides. J. Phys. Chem. Ref. Data 2014, 43, 013101. [Google Scholar] [CrossRef] [Green Version]
- Zinkevich, M. Thermodynamic Database for Rare Earth Sesquioxides. Available online: https://materialsdata.nist.gov/handle/11256/965 (accessed on 16 April 2020).
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Eyring, L. Chapter 27 The binary rare earth oxides. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1979; Volume 3, pp. 337–399. [Google Scholar]
- Foex, M. Study on yttrium oxide melting point. High Temp. High Press. 1977, 9, 269–282. [Google Scholar]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, F.; Chen, S.; Cao, W. Computational Thermodynamics Aided High-Entropy Alloy Design. Jom 2012, 64, 839–845. [Google Scholar] [CrossRef]
- Zhong, Y.; Sabarou, H.; Yan, X.; Yang, M.; Gao, M.C.; Liu, X.; Sisson, R.D., Jr. Exploration of high entropy ceramics (HECs) with computational thermodynamics—A case study with LaMnO3±δ. Mater. Des. 2019, 182, 108060. [Google Scholar] [CrossRef]
- Gong, W.; Liu, Y.; Xie, Y.; Zhao, Z.; Ushakov, S.V.; Navrotsky, A. Thermodynamic assessment of BaO–Ln2O3 (Ln = La, Pr, Eu, Gd, Er) systems. J. Am. Ceram. Soc. 2020, 103, 3896–3904. [Google Scholar] [CrossRef]
- Gong, W.; Ushakov, S.V.; Agca, C.; Navrotsky, A. Thermochemistry of BaSm2O4 and thermodynamic assessment of the BaO–Sm2O3 system. J. Am. Ceram. Soc. 2018, 101, 5827–5835. [Google Scholar] [CrossRef]
- Roth, R.S.S.; Schneider, S.J. Phase Equilibria in Systems Involving the Rare-Earth Oxides. Part I. Polymorphism of the Oxides of the Trivalent Rare-Earth Ions. J. Res. Natl. Bur. Stand. A Phys. Chem. 1960, 64, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, A.; Coutures, J.; Foex, M. High-temperature phase diagram of the lanthanum(III) oxide-ytterbium(III) oxide system. J. Solid State Chem. 1972, 4, 219–222. [Google Scholar] [CrossRef]
- Maister, I.M.; Lopato, L.M.; Shevchenko, A.V.; Nigmanov, B.S. Yttrium oxide-erbium oxide system. Izv. Akad. Nauk SSSR Neorg. Mater. 1984, 20, 446–448. [Google Scholar]
- Norgren, S. Thermodynamic assessment of the Ho-Tb, Ho-Dy, Ho-Er, Er-Tb, and Er-Dy systems. J. Phase Equilibria 2000, 21, 148–156. [Google Scholar] [CrossRef]
- Okamoto, H.; Massalski, T.B. Thermodynamically improbable phase diagrams. J. Phase Equilibria 1991, 12, 148–168. [Google Scholar] [CrossRef] [Green Version]
- Spedding, F.H.; Sanden, B.; Beaudry, B.J. Erbium-yttrium, terbium-holmium, terbium-erbium, dysprosium-holmium, dysprosium-erbium, and holmium-erbium phase systems. J. Less-Common Met. 1973, 31, 1–14. [Google Scholar] [CrossRef]
- Hoekstra, H.R. Phase relationships in the rare earth sesquioxides at high pressure. Inorg. Chem. 1966, 5, 754–757. [Google Scholar] [CrossRef]
- Bai, L.; Liu, J.; Li, X.; Jiang, S.; Xiao, W.; Li, Y.; Tang, L.; Zhang, Y.; Zhang, D. Pressure-induced phase transformations in cubic Gd2O3. J. Appl. Phys. 2009, 106, 073507. [Google Scholar] [CrossRef]
- Irshad, K.A.; Anees, P.; Sahoo, S.; Sanjay Kumar, N.R.; Srihari, V.; Kalavathi, S.; Chandra Shekar, N.V. Pressure induced structural phase transition in rare earth sesquioxide Tm2O3: Experiment and ab initio calculations. J. Appl. Phys. 2018, 124, 155901. [Google Scholar] [CrossRef]
- Liu, D.; Lei, W.; Li, Y.; Ma, Y.; Hao, J.; Chen, X.; Jin, Y.; Liu, D.; Yu, S.; Cui, Q.; et al. High-Pressure Structural Transitions of Sc2O3 by X-ray Diffraction, Raman Spectra, and Ab Initio Calculations. Inorg. Chem. 2009, 48, 8251–8256. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D. Thermal expansion data: III. Sesquioxides, M2O3, with the corundum and the A-, B- and C-M2O3 structures. Br. Ceram. Trans. J. 1984, 83, 92–98. [Google Scholar]
- Ploetz, G.L.; Krystyniak, C.W.; Dumas, H.E. Sintering characteristics of rare-earth oxides. J. Am. Ceram. Soc. 1958, 41, 551–554. [Google Scholar] [CrossRef]
- Coutures, J.P.; Rand, M.H. Melting temperatures of refractory oxides: Part II. Lanthanoid sesquioxides. Pure Appl. Chem. 1989, 61, 1461–1482. [Google Scholar] [CrossRef]
- Nakanishi, B.R.; Allanore, A. Electrochemical Investigation of Molten Lanthanum-Yttrium Oxide for Selective Liquid Rare-Earth Metal Extraction. J. Electrochem. Soc. 2019, 166, E420–E428. [Google Scholar] [CrossRef]
- Rouanet, A. Zirconium dioxide—Lanthanide oxide systems close to the melting point. Rev. Int. Hautes Temp. Refract. 1971, 8, 161–180. [Google Scholar]
- Fabrichnaya, O.; Savinykh, G.; Zienert, T.; Schreiber, G.; Seifert, H.J. Phase relations in the ZrO2-Sm2O3-Y2O3-Al2O3 system: Experimental investigation and thermodynamic modelling. Int. J. Mater. Res. 2012, 103, 1469–1487. [Google Scholar] [CrossRef]
- Shevchenko, A.V.; Nigmanov, B.S.; Zajtseva, Z.A.; Lopato, L.M. Interaction of samarium and gadolinium oxdes with yttrium oxides. Izv. Akad. Nauk SSSR Neorg. Mater. 1986, 22, 775–779. [Google Scholar]





| Rare Earth | HE-Nd | HE-Gd | HE-Y |
|---|---|---|---|
| La | 19.5 ± 0.2 | 18.7 ± 0.4 | 17.7 ± 0.8 |
| Sm | 20.1 ± 0.1 | 20.7 ± 0.1 | 19.8 ± 0.1 |
| Dy | 20.8 ± 0.1 | 21.1 ± 0.2 | 18.0 ± 0.3 |
| Er | 20.3 ± 0.1 | 20.3 ± 0.2 | 18.4 ± 0.3 |
| Y | - | - | 26.0 ± 0.4 |
| Gd | - | 19.3 ± 0.2 | - |
| Nd | 19.3 ± 0.1 | - | - |
| MW g/mol | 350.89 | 358.84 | 323.09 |
| Ave radii 1 Å | 0.954 | 0.945 | 0.936 |
| Experiment | Phase | HE-Nd | HE-Gd | HE-Y |
|---|---|---|---|---|
| Air 800 °C 96 h | C-Type (Cubic, Bixbyite-Type) Ia-3, Z = 16 | |||
| a, Å | 10.903(1) | 10.863(2) | 10.814(3) | |
| V, A3/z | 81.0(1) | 80.1(1) | 79.0(1) | |
| Size | 38 ± 1 nm | 21 ± 1 nm | 21 ± 1 nm | |
| wt.% | ~85 wt.% | ~90 wt.% | 100 wt.% | |
| B-Type (Monoclinic, Sm2O3-Type) C2/m, Z = 6 | ||||
| a, Å | 14.259(5) | 13.90(4) | - | |
| b, Å | 3.620(1) | 3.53(5) | ||
| c, Å | 8.862(3) | 9.00(4) | ||
| β, ° | 100.67(1) | 96.8(1) | ||
| V, A3/z | 74.9 ± 0.1 | 73 ± 2 | ||
| Size | 32 ± 2 nm | 13 ± 2 nm | ||
| wt.% | ~15 wt.% | ~10 wt.% | ||
| V (C→B) % | −7.5 ± 0.1% | −9.7 ± 0.3% | ||
| Air 1100 °C 12 h | C-type (cubic, bixbyite-type) Ia-3, Z = 16 | |||
| a, Å | 10.825(1) | 10.804(1) | ||
| V, A3/z | - | 79.0(1) | 78.8(1) | |
| Size | 49 ±1 nm | 65 ± 1 nm | ||
| wt.% | ~30 wt.% | 100 wt.% | ||
| B-type (monoclinic, Sm2O3-type) C2/m, Z = 6 | ||||
| a, Å | 14.242(1) | 14.227(1) | ||
| b, Å | 3.6152(2) | 3.601(1) | ||
| c, Å | 8.857(1) | 8.833(1) | - | |
| β, ° | 100.62(4) | 100.66(1) | ||
| V, A3/z | 74.7(1) | 74.1(1) | ||
| Size | 73 ± 1 nm | 68 ± 1 nm | ||
| wt.% | ~70 wt.% | |||
| V (C→B) % | −8.7 ± 0.1% | −6.8 ± 0.1% | ||
| Unit Cell | HE-Nd * | HE-Gd | HE-Y | |||
|---|---|---|---|---|---|---|
| Parameters | Splat Quench | 800 °C/60 d | Splat Quench | 800 °C/60 d | Splat Quench | 800 °C/60 d |
| a, Å | 14.245(1) | 14.244(1) | 14.194(1) | 14.180(1) | 14.159(2) | 14.139(1) |
| b, Å | 3.6150(1) | 3.6025(1) | 3.5956(1) | 3.5840(1) | 3.5741(2) | 3.5617(1) |
| c, Å | 8.857(1) | 8.839(1) | 8.818(1) | 8.797(1) | 8.781(1) | 8.758(1) |
| β, ° | 100.63(1) | 100.69(1) | 100.59(1) | 100.59(1) | 100.61(1) | 100.65(1) |
| V, A3/z | 74.72(1) | 74.28(1) | 73.73(1) | 73.24(1) | 72.79(1) | 72.24(1) |
| Cryst. size | 76 ± 1 nm | 95 ± 1 nm | 80 ± 1 nm | 101 ± 1 nm | 86 ± 1 nm | 147 ± 3 nm |
| HE-Nd | HE-Gd | HE-Y | |
|---|---|---|---|
| TB-A °C | 1916 ± 9(5) * | 1957 ± 5(4) | 1975 ± 13(4) |
| HB-A J/g | 57 ± 3(10) | 56 ± 7(8) | 56 ± 8(8) |
| HB-A kJ/mol | 19.8 ± 1.0 | 20.3 ± 2.7 | 18.0 ± 2.7 |
| SB-A J/mol/K | 9.0 ± 0.1 | 9.1 ± 0.1 | 8.0 ± 0.2 |
| TA-H °C | 2125 ± 3(5) | 2180 ± 2(4) | 2199 ± 4(6) |
| HA-H J/g | 22 ± 3(10) | 23 ± 1(7) | 28 ± 1(9) |
| HA-H kJ/mol | 7.7 ± 0.9 | 8.3 ± 0.5 | 9.2 ± 0.3 |
| SA-H J/mol/K | 3.2 ± 0.1 | 3.4 ± 0.1 | 3.7 ± 0.1 |
| TH-X °C | 2202 ± 4(2) | 2235 ± 5(2) | 2254 ± 8(4) |
| HH-X J/g | 79 ± 1(2) | 85 ± 23(3) | 126 ± 7(4) |
| HH-X kJ/mol | 27.8 ± 0.2 | 30.6 ± 8.3 | 40.6 ± 2.4 |
| SH-X J/mol/K | 11.2 ± 0.1 | 12.2 ± 0.4 | 16.1 ± 0.1 |
| Tm °C | 2456 ± 12 |
| Structure/Sample | HE-Nd | HE-Gd | HE-Y | |
|---|---|---|---|---|
| Ttr (DTA) | TB-A, °C | 1916 ± 9 | 1957 ± 5 | 1975 ± 13 |
| B-type monoclinic C2/m, Z = 6 | a, Å | 14.433(8) | 14.403(5) | 14.367(7) |
| b, Å | 3.711(1) | 3.697(1) | 3.674(1) | |
| c, Å | 9.026(5) | 8.987(2) | 8.965(3) | |
| β, ° | 101.35(2) | 101.18(1) | 101.09(2) | |
| V, A3/z | 79.0(2) | 78.2(1) | 77.4(1) | |
| A-type trigonal P-3m1, Z = 1 | a, Å | 3.885(1) | 3.874(1) | 3.864(1) |
| c, Å | 6.199(1) | 6.175(2) | 6.171(1) | |
| V, A3/z | 81.03(2) | 80.24(2) | 79.78(1) | |
| V (B→A), % | −2.5 ± 0.1 | −2.5 ± 0.1 | −3.1 ± 0.1 | |
| Ttr (DTA) | TH-X, °C | 2202 ± 4 | 2235 ± 5 | 2254 ± 8 |
| H-type P63/mmc, Z = 1 | a, Å | 3.898(1) | 3.869(1) | |
| c, Å | 6.216(1) | 6.168(1) | ||
| V, A3/z | 81.81(1) | 79.96(1) | ||
| X-type Im-3m, Z = 1 | a, Å | 4.324(1) | 4.2989(1) | |
| V, A3/z | 80.85(2) | 79.44(1) | ||
| V (H→X), % | −1.2 ± 0.1 | −0.6 ± 0.1 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushakov, S.V.; Hayun, S.; Gong, W.; Navrotsky, A. Thermal Analysis of High Entropy Rare Earth Oxides. Materials 2020, 13, 3141. https://doi.org/10.3390/ma13143141
Ushakov SV, Hayun S, Gong W, Navrotsky A. Thermal Analysis of High Entropy Rare Earth Oxides. Materials. 2020; 13(14):3141. https://doi.org/10.3390/ma13143141
Chicago/Turabian StyleUshakov, Sergey V., Shmuel Hayun, Weiping Gong, and Alexandra Navrotsky. 2020. "Thermal Analysis of High Entropy Rare Earth Oxides" Materials 13, no. 14: 3141. https://doi.org/10.3390/ma13143141
APA StyleUshakov, S. V., Hayun, S., Gong, W., & Navrotsky, A. (2020). Thermal Analysis of High Entropy Rare Earth Oxides. Materials, 13(14), 3141. https://doi.org/10.3390/ma13143141