Synthesis of Carbon Nanomaterials from Biomass Utilizing Ionic Liquids for Potential Application in Solar Energy Conversion and Storage
Abstract
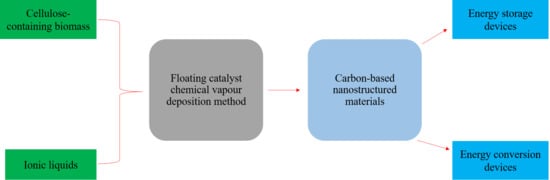
:1. Introduction
2. Carbon-Based Nanostructured Materials
2.1. Synthesis of Carbon-Based Nanostructured Materials
2.2. Biomass as a Renewable Carbon Source for CNM Synthesis
2.2.1. Biomass Dissolution
2.2.2. Ionic Liquids in Dissolution of Biomass
2.3. Biomass-Derived Carbon-Based Nanostructured Materials
2.3.1. Graphene
2.3.2. Carbon Nanotubes (CNTs)
2.3.3. Carbon Nanofibers (CNFs)
2.3.4. Carbon Onions
2.3.5. Carbon Spheres
3. Surface Modifications of CNMs
4. Application of Biomass-Derived Carbon-Based Nanostructured Materials in Energy-Related Devices
4.1. Energy Conversion: Solar Cells
4.1.1. Organic Solar Cells
4.1.2. Hybrid Solar Cells
4.2. Energy Storage
4.2.1. Batteries
4.2.2. Supercapacitors
5. Summary and Key Findings
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O′Brien, S.C.; Curl, R.F.; Smalley, R.E. C 60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Kim, H.-S.; Kim, J.H.; Shin, U.S.; Lee, S.-H. Carbon nanotube nanocomposites with highly enhanced strength and conductivity for flexible electric circuits. Langmuir 2015, 31, 7844–7851. [Google Scholar] [CrossRef] [PubMed]
- Gspann, T.S.; Juckes, S.M.; Niven, J.F.; Johnson, M.B.; Elliott, J.A.; White, M.A.; Windle, A.H. High thermal conductivities of carbon nanotube films and micro-fibres and their dependence on morphology. Carbon 2017, 114, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.T.; Vijayan, B.K.; Lyandres, O.; Gray, K.A.; Hersam, M.C. Effect of dimensionality on the photocatalytic behavior of carbon–titania nanosheet composites: Charge transfer at nanomaterial interfaces. J. Phys. Chem. Lett. 2012, 3, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem. Commun. 2014, 50, 6818–6830. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Guldi, D.M.; Sgobba, V. Carbon nanostructures for solar energy conversion schemes. Chem. Commun. 2011, 47, 606–610. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Webster, T.J. Carbon nanostructures for orthopedic medical applications. Nanomedicine 2011, 6, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, I.; Das, S.; Kang, C.; Choi, W. Application of carbon nanostructures—Energy to electronics. JOM 2011, 63, 70–76. [Google Scholar] [CrossRef]
- Mhlanga, S.D.; Coville, N.J.; Iyuke, S.E.; Afolabi, A.S.; Abdulkareem, A.S.; Kunjuzwa, N. Controlled syntheses of carbon spheres in a swirled floating catalytic chemical vapour deposition vertical reactor. J. Exp. Nanosci. 2010, 5, 40–51. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Recent progress in fabrication, structure, and properties of carbon fibers. Polym. Rev. 2012, 52, 234–258. [Google Scholar] [CrossRef]
- Stamatin, I.; Morozan, A.; Dumitru, A.; Vulpe, S. Highly oriented carbon ribbons for advanced multifunctional material engineering. Fuller. Nanotub. Carbon Nanostruct. 2005, 13, 543–551. [Google Scholar] [CrossRef]
- Mugadza, K.; Nyamori, V.O.; Mola, G.T.; Simoyi, R.H.; Ndungu, P.G. Low temperature synthesis of multiwalled carbon nanotubes and incorporation into an organic solar cell. J. Exp. Nanosci. 2017, 12, 363–383. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Li, F.; Yang, Q.; Cheng, H.-M.; Bai, J. Influence of ferrocene/benzene mole ratio on the synthesis of carbon nanostructures. Chem. Phys. Lett. 2003, 376, 83–89. [Google Scholar] [CrossRef]
- Nyamori, V.O.; Coville, N.J. Effect of ferrocene/carbon ratio on the size and shape of carbon nanotubes and microspheres. Organomet 2007, 26, 4083–4085. [Google Scholar] [CrossRef]
- Sevilla, M.; Salinas Martínez-de Lecea, C.; Valdés-Solís, T.; Morallón, E.; Fuertes, A.B. Solid-phase synthesis of graphitic carbon nanostructures from iron and cobalt gluconates and their utilization as electrocatalyst supports. Phys. Chem. Chem. Phys. 2008, 10, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Mutuma, B.K.; Matsoso, B.; Ranganathan, K.; Wamwangi, D.; Coville, N.J. Generation of open-ended, worm-like and graphene-like structures from layered spherical carbon materials. RSC Adv. 2016, 6, 20399–20408. [Google Scholar] [CrossRef] [Green Version]
- Kairi, M.I.; Khavarian, M.; Bakar, S.A.; Vigolo, B.; Mohamed, A.R. Recent trends in graphene materials synthesized by CVD with various carbon precursors. J. Mater. Sci. 2018, 53, 851–879. [Google Scholar] [CrossRef]
- Keru, G.; Ndungu, P.G.; Nyamori, V.O. Nitrogen-doped carbon nanotubes synthesised by pyrolysis of (4-{[(pyridine-4-yl)methylidene]amino}phenyl)ferrocene. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mat. Sci. Semicon. Proc. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Growth of nanotubes using IC-PECVD as benzene carbon carrier. Microsyst Technol. 2017, 23, 5447–5453. [Google Scholar] [CrossRef]
- Yadav, M.D.; Dasgupta, K.; Patwardhan, A.W.; Joshi, J.B. High performance fibers from carbon nanotubes: Synthesis, characterization, and applications in composites—A review. Ind. Eng. Chem. Res. 2017, 56, 12407–12437. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Bridge, S.A. A review of biomass pyrolysis and pyrolysis technologies. In Biomass Pyrolysis Liquids Upgrading and Utilization; Bridgwater, A.V., Grassi, G., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 11–92. [Google Scholar]
- Zhu, M.; Diao, G. Review on the progress in synthesis and application of magnetic carbon nanocomposites. Nanoscale 2011, 3, 2748–2767. [Google Scholar] [CrossRef]
- Brunori, G. Biomass, biovalue and sustainability: Some thoughts on the definition of the bioeconomy. EuroChoices 2013, 12, 48–52. [Google Scholar] [CrossRef]
- Harris, D.; DeBolt, S. Relative crystallinity of plant biomass: Studies on assembly, adaptation and acclimation. PLoS ONE 2008, 3, e2897. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Louis, H.; Temitope, H.A.; Philip, M.; Amos, P.I.; Magu, T.O.; Ozioma, A.U.; Amusan, O.O. Ionic liquids (ILs): Advances in biorefinery for the efficient conversion of lignocellulosic biomass. Asian J. Green Chem. 2019, 3, 391–417. [Google Scholar]
- Rieland, J.M.; Love, B.J. Ionic liquids: A milestone on the pathway to greener recycling of cellulose from biomass. Resour. Conserv. Recycl. 2020, 155, 104678. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Weldemhret, T.G.; Bañares, A.B.; Ramos, K.R.M.; Lee, W.-K.; Nisola, G.M.; Valdehuesa, K.N.G.; Chung, W.-J. Current advances in ionic liquid-based pretreatment and depolymerization of macroalgal biomass. Renew. Energy 2020, 152, 283–299. [Google Scholar] [CrossRef]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilibria 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Gibril, M.E.; Yue, Z.; Da, L.X.; Huan, L.; Xuan, Z.; Feng, L.H.; Muhuo, Y. Current status of applications of ionic liquids for cellulose dissolution and modifications: Review. Int. J. Eng. Sci. Technol. 2012, 4, 3556–3571. [Google Scholar]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, L.; Qin, X.; Li, Y. Carbon nanomaterials for photovoltaic process. Nano Energy 2015, 15, 490–522. [Google Scholar] [CrossRef]
- Li, J.; Yun, S.; Han, F.; Si, Y.; Arshad, A.; Zhang, Y.; Chidambaram, B.; Zafar, N.; Qiao, X. Biomass-derived carbon boosted catalytic properties of tungsten-based nanohybrids for accelerating the triiodide reduction in dye-sensitized solar cells. J. Colloid Interface Sci. 2020, 578, 184–194. [Google Scholar] [CrossRef]
- Biswal, M.; Banerjee, A.; Deo, M.; Ogale, S. From dead leaves to high energy density supercapacitors. Energy Environ. Sci. 2013, 6, 1249–1259. [Google Scholar] [CrossRef]
- Dresselhaus, M. The wonderful world of carbon. In Supercarbon; Springer: Berlin/Heidelberg, Germany, 1998; pp. 9–29. [Google Scholar]
- Keru, G.; Ndungu, P.G.; Mola, G.T.; Nogueira, A.F.; Nyamori, V.O. Organic solar cells with boron- or nitrogen-doped carbon nanotubes in the P3HT: PCBM photoactive layer. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Mombeshora, E.T.; Ndungu, P.G.; Nyamori, V.O. The physicochemical properties and capacitive functionality of pyrrolic- and pyridinic-nitrogen, and boron-doped reduced graphene oxide. Electrochim. Acta 2017, 258, 467–476. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Sarapuu, A.; Kook, M.; Rauwel, P.; Saar, R.; Aruväli, J.; Tammeveski, K. Electrocatalysis of oxygen reduction on iron- and cobalt-containing nitrogen-doped carbon nanotubes in acid media. Electrochim. Acta 2016, 218, 303–310. [Google Scholar] [CrossRef]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J.; Elías, A.L.; Muñoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.-C.; et al. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Bartelmess, J.; Giordani, S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Daems, N.; Sheng, X.; Vankelecom, I.F.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Mombeshora, E.T.; Nyamori, V.O. A review on the use of carbon nanostructured materials in electrochemical capacitors. Int. J. Energy Res. 2015, 39, 1955–1980. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Yudasaka, M.; Ichihashi, T.; Komatsu, T.; Iijima, S. Single-wall carbon nanotubes formed by a single laser-beam pulse. Chem. Phys. Lett. 1999, 299, 91–96. [Google Scholar] [CrossRef]
- Rafique, M.M.A.; Iqbal, J. Production of carbon nanotubes by different routes-a review. JEAS 2011, 1, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Zaikovskii, A.; Novopashin, S.; Maltsev, V.; Kardash, T.; Shundrina, I. Tin–carbon nanomaterial formation in a helium atmosphere during arc-discharge. RSC Adv. 2019, 9, 36621–36630. [Google Scholar] [CrossRef]
- Zhao, S.; Hong, R.; Luo, Z.; Lu, H.; Yan, B. Carbon nanostructures production by AC arc discharge plasma process at atmospheric pressure. J. Nanomater. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Morales, A.M.; Lieber, C.M. A laser ablation method for the synthesis of crystalline semiconductor nanowires. Science 1998, 279, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, E.N.; Nyamori, V.O.; Coville, N.J. CVD synthesis of nitrogen doped carbon nanotubes using ferrocene/aniline mixtures. J. Organomet. Chem. 2008, 693, 2942–2948. [Google Scholar] [CrossRef]
- Öncel, Ç.; Yürüm, Y. Carbon nanotube synthesis via the catalytic CVD method: A review on the effect of reaction parameters. Fuller. Nanotub. Carbon Nanostruct. 2006, 14, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A review of carbon nanomaterials’ synthesis via the chemical vapor deposition (CVD) method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Plutnar, J.; Pumera, M.; Sofer, Z. The chemistry of CVD graphene. J. Mat. Chem. C 2018, 6, 6082–6101. [Google Scholar] [CrossRef]
- Ombaka, L.M.; Ndungu, P.G.; Nyamori, V.O. Tuning the nitrogen content and surface properties of nitrogen-doped carbon nanotubes synthesized using a nitrogen-containing ferrocenyl derivative and ethylbenzoate. J. Mater. Sci. 2015, 50, 1187–1200. [Google Scholar] [CrossRef]
- Panickar, R.; Sobhan, C.B.; Chakravorti, S. Chemical vapor deposition synthesis of carbon spheres: Effects of temperature and hydrogen. Vacuum 2020, 172, 109108. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. J. Geophys. Res. Solid Earth 2014, 5, 477–483. [Google Scholar]
- Petrov, N.; Budinova, T.; Razvigorova, M.; Zanzi, R.; Björnbom, E.; Minkova, V. Preparation of activated carbons from cherry stones, apricot stones and grape seeds for removal of metal ions from water. In Proceedings of the 2nd OlleIndstorm Symposium on Renewable Energy-Bioenergy, Stockholm, Sweden, 1 July 1999; pp. 9–11. [Google Scholar]
- Joshi, P. Novel counter electrodes of dye-sensitized solar cells based on activated carbon prepared from wood of Choerospondias axillaris seed-stones and Alnus nepalensis plant. Int. J. Adv. Res. Technol. 2017, 3, 8–11. [Google Scholar]
- Guo, J.; Zhang, J.; Jiang, F.; Zhao, S.; Su, Q.; Du, G. Microporous carbon nanosheets derived from corncobs for lithium–sulfur batteries. Electrochim. Acta 2015, 176, 853–860. [Google Scholar] [CrossRef]
- Ma, G.; Yang, Q.; Sun, K.; Peng, H.; Ran, F.; Zhao, X.; Lei, Z. Nitrogen-doped porous carbon derived from biomass waste for high-performance supercapacitor. Bioresour. Technol. 2015, 197, 137–142. [Google Scholar] [CrossRef]
- Prasek, J.; Drbohlavova, J.; Chomoucka, J.; Hubalek, J.; Jasek, O.; Adam, V.; Kizek, R. Methods for carbon nanotubes synthesis—Review. J. Mater. Chem. 2011, 21, 15872–15884. [Google Scholar] [CrossRef]
- Coleman, K.S. Nanotubes. Annu. Rep. Prog. Chem. Sect. A Inorg. Chem. 2012, 108, 478–492. [Google Scholar] [CrossRef]
- Wirth, C.T.; Bayer, B.C.; Gamalski, A.D.; Esconjauregui, S.; Weatherup, R.S.; Ducati, C.; Baehtz, C.; Robertson, J.; Hofmann, S. The phase of iron catalyst nanoparticles during carbon nanotube growth. Chem. Mater. 2012, 24, 4633–4640. [Google Scholar] [CrossRef]
- Bati, A.S.; Yu, L.; Batmunkh, M.; Shapter, J.G. Synthesis, purification, properties and characterization of sorted single-walled carbon nanotubes. Nanoscale 2018, 10, 22087–22139. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Ding, F. Chirality-controlled synthesis of single-walled carbon nanotubes—From mechanistic studies toward experimental realization. Mater. Today Commun. 2018, 21, 845–860. [Google Scholar] [CrossRef]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; Chai, Z.; Hu, W. Low-temperature plasma synthesis of carbon nanotubes and graphene based materials and their fuel cell applications. Chem. Soc. Rev. 2013, 42, 8821–8834. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2018, 141, 467–480. [Google Scholar] [CrossRef]
- Conroy, D.; Moisala, A.; Cardoso, S.; Windle, A.; Davidson, J. Carbon nanotube reactor: Ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up. Chem. Eng. Sci. 2010, 65, 2965–2977. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Whelan, C.M.; Maex, K. The reasons why metals catalyze the nucleation and growth of carbon nanotubes and other carbon nanomorphologies. Carbon 2009, 47, 659–669. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [Green Version]
- Kiang, C.-H. Carbon rings and cages in the growth of single-walled carbon nanotubes. J. Chem. Phys. 2000, 113, 4763–4766. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J.; Jeong, A.Y. Catalyst effect on carbon nanotubes synthesized by thermal chemical vapor deposition. Chem. Phys. Lett. 2002, 360, 250–255. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J.; Bensakhria, A.; Valette, J. Influence of impregnated metal on the pyrolysis conversion of biomass constituents. J. Anal. Appl. Pyrolysis 2012, 95, 213–226. [Google Scholar] [CrossRef]
- Lee, J.S.; Wang, X.; Luo, H.; Dai, S. Fluidic carbon precursors for formation of functional carbon under ambient pressure based on ionic liquids. Adv. Mater. 2010, 22, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, D.; Qian, W.; Zhang, J.; Yan, F. Heteroatom-containing porous carbons derived from ionic liquid-doped alkali organic salts for supercapacitors. Small 2016, 12, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Goodell, B.; Xie, X.; Qian, Y.; Daniel, G.; Peterson, M.; Jellison, J. Carbon nanotubes produced from natural cellulosic materials. J. Nanosci. Nanotechnol. 2008, 8, 2472–2474. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Ostrikov, K. Sustainable life cycles of natural-precursor-derived nanocarbons. Chem. Rev. 2016, 116, 163–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, J.; Luo, C.; Cong, Q.; Yuan, X. Carbon nanotubes and Cu–Zn nanoparticles synthesis using hyperaccumulator plants. Environ. Chem. Lett. 2012, 10, 153–158. [Google Scholar] [CrossRef]
- Watt, W.; Perov, B. Strong Fibres, Handbook of composites Vol. 1; Nelly, A., Rabotnov, Y.N., Eds.; Elsevier Science Ltd: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [Green Version]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- 95 Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740–1763. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Xu, L.; Chen, M.; Wang, L.; Liu, J.; Han, S.; Mei, C.; Zhong, Q. The fabrication of bio-renewable and recyclable cellulose based carbon microspheres incorporated by CoFe2O4 and the photocatalytic properties. J. Clean. Prod. 2018, 196, 594–603. [Google Scholar] [CrossRef]
- Bai, Q.; Xiong, Q.; Li, C.; Shen, Y.; Uyama, H. Hierarchical porous carbons from a sodium alginate/bacterial cellulose composite for high-performance supercapacitor electrodes. Appl. Surf. Sci. 2018, 455, 795–807. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Graphitic carbon nanostructures from cellulose. Chem. Phys. Lett. 2010, 490, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, L.; Naboka, O.; Ogenko, V.; Gatenholm, P.; Enoksson, P. One-pot synthesis of carbon nanotubes from renewable resource: Cellulose acetate. J. Mater. Sci. 2014, 49, 1144–1149. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T. Unconventional methods in cellulose functionalization. Prog. Polym. Sci. 2001, 26, 1689–1762. [Google Scholar] [CrossRef]
- Swatloski, R.; Rogers, R.; Holbrey, J. Dissolution and Processing of Cellulose Using Ionic Liquids. U.S. Patent 6,824,599, 30 November 2004. [Google Scholar]
- Zhu, S.; Wu, Y.; Chen, Q.; Yu, Z.; Wang, C.; Jin, S.; Ding, Y.; Wu, G. Dissolution of cellulose with ionic liquids and its application: A mini-review. Green Chem. 2006, 8, 325–327. [Google Scholar] [CrossRef]
- Graenacher, C. Cellulose Solution. U.S. Patent 1,943,176, 9 January 1934. [Google Scholar]
- Fink, H.-P.; Weigel, P.; Purz, H.; Ganster, J. Structure formation of regenerated cellulose materials from NMMO-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- McCormick, C.L.; Dawsey, T.R. Preparation of cellulose derivatives via ring-opening reactions with cyclic reagents in lithium chloride/N, N-dimethylacetamide. Macromolecules 1990, 23, 3606–3610. [Google Scholar] [CrossRef]
- Tamai, N.; Tatsumi, D.; Matsumoto, T. Rheological properties and molecular structure of tunicate cellulose in LiCl/1, 3-dimethyl-2-imidazolidinone. Biomacromolecules 2004, 5, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Ciacco, G.T.; Liebert, T.F.; Frollini, E.; Heinze, T.J. Application of the solvent dimethyl sulfoxide/tetrabutyl-ammonium fluoride trihydrate as reaction medium for the homogeneous acylation of Sisal cellulose. Cellulose 2003, 10, 125–132. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 8, 405–422. [Google Scholar]
- Rebelo, L.P.N.; Canongia Lopes, J.N.; Esperança, J.M.S.S.; Filipe, E. On the critical temperature, normal boiling point, and vapor pressure of ionic liquids. J. Phys. Chem. B 2005, 109, 6040–6043. [Google Scholar] [CrossRef]
- Eike, D.M.; Brennecke, J.F.; Maginn, E.J. Predicting melting points of quaternary ammonium ionic liquids. Green Chem. 2003, 5, 323–328. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J. Am. Chem. Soc. Dalton Trans. 1999, 2133–2139. [Google Scholar] [CrossRef]
- Suarez, P.A.Z.; Einloft, S.; Dullius, J.E.L.; De Souza, R.F.; Dupont, J. Synthesis and physical-chemical properties of ionic liquids based on 1- n-butyl-3-methylimidazolium cation. J. Chim. Phys. Phys. Chim. Biol. 1998, 95, 1626–1639. [Google Scholar] [CrossRef]
- Freemantle, M. Designer solvents. Chem. Eng. News 1998, 76, 32–37. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green solvents for the future. Pure Appl. Chem. 2000, 72, 1391–1398. [Google Scholar] [CrossRef] [Green Version]
- Endres, F.; Zein El Abedin, S. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic Liquids—New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley Online Library: Boschstraße, Germany, 2008; Volume 1. [Google Scholar]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, N.; Thalangamaarachchige, V.D.; Troxell, S.; Quitevis, E.L.; Abidi, N. Substituent effects on cellulose dissolution in imidazolium-based ionic liquids. Cellulose 2018, 25, 6887–6900. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of anionic structure on the dissolution of cellulose in ionic liquids revealed by molecular simulation. Carbohydr. Polym. 2013, 94, 723–730. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Stark, A. Ionic liquids in the biorefinery: A critical assessment of their potential. Energy Environ. Sci. 2011, 4, 19–32. [Google Scholar] [CrossRef]
- Fukaya, Y.; Sugimoto, A.; Ohno, H. Superior solubility of polysaccharides in low viscosity, polar, and halogen-free 1,3-dialkylimidazolium formates. Biomacromolecules 2006, 7, 3295–3297. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Yang, D.; Sun, Q.; Liu, Y.; Zhao, H. Lignocellulose aerogel from wood-ionic liquid solution (1-allyl-3-methylimidazolium chloride) under freezing and thawing conditions. Biomacromolecules 2011, 12, 1860–1867. [Google Scholar] [CrossRef]
- Lovell, C.S.; Walker, A.; Damion, R.A.; Radhi, A.; Tanner, S.F.; Budtova, T.; Ries, M.E. Influence of cellulose on ion diffusivity in 1-ethyl-3-methyl-imidazolium acetate cellulose solutions. Biomacromolecules 2010, 11, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Wang, J.; Zhang, S. Effects of cationic structure on cellulose dissolution in ionic liquids: A molecular dynamics study. Chem. Phys. Chem. 2012, 13, 3126–3133. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Z.-L. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Skoda, M.; Dudek, I.; Jarosz, A.; Szukiewicz, D. Graphene: One material, many possibilities—Application difficulties in biological systems. J. Nanomater. 2014, 2014, 890246. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abergel, D.S.L.; Apalkov, V.; Berashevich, J.; Ziegler, K.; Chakraborty, T. Properties of graphene: A theoretical perspective. Adv. Phys. 2010, 59, 261–482. [Google Scholar]
- Mombeshora, E.T.; Ndungu, P.G.; Nyamori, V.O. Effect of graphite/sodium nitrate ratio and reaction time on the physicochemical properties of graphene oxide. New Carbon Mater. 2017, 32, 174–187. [Google Scholar] [CrossRef]
- Primo, A.; Atienzar, P.; Sanchez, E.; Delgado, J.M.; Garcia, H. From biomass wastes to large-area, high-quality, N-doped graphene: Catalyst-free carbonization of chitosan coatings on arbitrary substrates. Chem. Commun. 2012, 48, 9254–9256. [Google Scholar] [CrossRef]
- Shams, S.S.; Zhang, L.S.; Hu, R.; Zhang, R.; Zhu, J. Synthesis of graphene from biomass: A green chemistry approach. Mater. Lett. 2015, 161, 476–479. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X. Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J. Electroanal. Chem. 2016, 768, 18–26. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Zhang, J.; Kou, Z.; Liu, X.; Asare, O.K.; Zhou, H.; Cheng, K.; Zhang, H.; Mai, L.; Pan, M.; et al. Self-organized 3D porous graphene dual-doped with biomass-sponsored nitrogen and sulfur for oxygen reduction and evolution. ACS Appl. Mater. Interfaces 2016, 8, 29408–29418. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Al-Marzouki, F.; Al-Ghamdi, A.A.; Abdel-Daiem, A. Different technical applications of carbon nanotubes. Nanoscale Res. Lett. 2015, 10, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical properties of carbon nanotube based fibers and their future use in electrical wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Chu, K.; Guo, H.; Jia, C.; Yin, F.; Zhang, X.; Liang, X.; Chen, H. Thermal properties of carbon nanotube–copper composites for thermal management applications. Nanoscale Res. Lett. 2010, 5, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.-M.; Timmermans, M.Y.; Tian, Y.; Nasibulin, A.G.; Kauppinen, E.I.; Kishimoto, S.; Mizutani, T.; Ohno, Y. Flexible high-performance carbon nanotube integrated circuits. Nat. Nanotech 2011, 6, 156. [Google Scholar] [CrossRef]
- Gharbavi, K.; Badehian, H. Optical properties of armchair (7, 7) single walled carbon nanotubes. AIP Adv. 2015, 5, 077155. [Google Scholar] [CrossRef] [Green Version]
- Marconnet, A.M.; Panzer, M.A.; Goodson, K.E. Thermal conduction phenomena in carbon nanotubes and related nanostructured materials. Rev. Mod. Phys. 2013, 85, 1295. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Pander, A.; Ishimoto, K.; Hatta, A.; Furuta, H. Significant decrease in the reflectance of thin CNT forest films tuned by the Taguchi method. Vacuum 2018, 154, 285–295. [Google Scholar] [CrossRef]
- Vivekanandhan, S.; Schreiber, M.; Muthuramkumar, S.; Misra, M.; Mohanty, A.K. Carbon nanotubes from renewable feedstocks: A move toward sustainable nanofabrication. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Luo, C.; Cong, Q. Synthesis of multiwalled carbon nanotubes/ZnO Nanocomposites using absorbent cotton. Nano-Micro Lett. 2011, 3, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.A.H.; Harrison, J.; Rooney, D.W. The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci. Rep. 2020, 10, 2563. [Google Scholar] [CrossRef] [PubMed]
- Bernd, M.G.S.; Bragança, S.R.; Heck, N.; Filho, L.C.P.d.S. Synthesis of carbon nanostructures by the pyrolysis of wood sawdust in a tubular reactor. J. Mater. Res. Technol. 2017, 6, 171–177. [Google Scholar] [CrossRef]
- Shi, K.; Yan, J.; Lester, E.; Wu, T. Catalyst-free synthesis of multiwalled carbon nanotubes via microwave-induced processing of biomass. Ind. Eng. Chem. 2014, 53, 15012–15019. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, J.; Kwong, F.L.; Ng, D.H.L.; Tjong, S.C. Synthesis of multiwalled carbon nanotubes from bamboo charcoal and the roles of minerals on their growth. Biomass Bioenerg 2012, 36, 12–19. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, E.; Mao, B.; Su, Z.; Chen, L.; Xu, L. Obtaining carbon nanotubes from grass. Nanotechnology 2005, 16, 1192. [Google Scholar] [CrossRef]
- Chen, L.-F.; Huang, Z.-H.; Liang, H.-W.; Gao, H.-L.; Yu, S.-H. Three-dimensional heteroatom-doped carbon nanofiber networks derived from bacterial cellulose for supercapacitors. Adv. Funct. Mater. 2014, 24, 5104–5111. [Google Scholar] [CrossRef]
- Liu, W.-J.; Tian, K.; Jiang, H. One-pot synthesis of Ni–NiFe2O4/carbon nanofiber composites from biomass for selective hydrogenation of aromatic nitro compounds. Green Chem. 2015, 17, 821–826. [Google Scholar] [CrossRef]
- Chen, L.-F.; Huang, Z.-H.; Liang, H.-W.; Yao, W.-T.; Yu, Z.-Y.; Yu, S.-H. Flexible all-solid-state high-power supercapacitor fabricated with nitrogen-doped carbon nanofiber electrode material derived from bacterial cellulose. Energy Environ. Sci. 2013, 6, 3331–3338. [Google Scholar] [CrossRef]
- Plonska-Brzezinska, M.E.; Echegoyen, L. Carbon nano-onions for supercapacitor electrodes: Recent developments and applications. J. Mater. Chem. A 2013, 1, 13703–13714. [Google Scholar] [CrossRef]
- Ghosh, M.; Sonkar, S.K.; Saxena, M.; Sarkar, S. Carbon nano-onions for imaging the life cycle of Drosophila Melanogaster. Small 2011, 7, 3170–3177. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, M.; Gan, L.; Zhao, Y.; Chen, L. Synthesis of micro- and mesoporous carbon spheres for supercapacitor electrode. J. Solid State Electrochem. 2013, 17, 2293–2301. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, X.; Zhu, B.; Liu, P.-F.; Lu, H.-T. Micro-mesoporous carbon spheres derived from carrageenan as electrode material for supercapacitors. J. Power Sources 2014, 268, 584–590. [Google Scholar] [CrossRef]
- Pari, G.; Darmawan, S.; Prihandoko, B. Porous carbon spheres from hydrothermal carbonization and KOH activation on cassava and tapioca flour raw material. Procedia Environ. Sci. 2014, 20, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-C.; Chang, X.; Liu, L.; Zhao, F.; Zhao, Y. Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem. 2010, 20, 1036–1052. [Google Scholar] [CrossRef]
- Karfa, P.; De, S.; Majhi, K.C.; Madhuri, R.; Sharma, P.K. 2.07-Functionalization of carbon nanostructures. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2019. [Google Scholar]
- He, C.-L.; Liu, R.; Li, D.-D.; Zhu, S.-E.; Wang, G.-W. Synthesis and functionalization of [60]fullerene-fused imidazolines. Org. Lett. 2013, 15, 1532–1535. [Google Scholar] [CrossRef]
- Yurovskaya, M.; Trushkov, I. Cycloaddition to buckminsterfullerene C60: Advancements and future prospects. Russ. Chem. Bull. 2002, 51, 367–443. [Google Scholar] [CrossRef]
- Liang, L.; Xie, W.; Fang, S.; He, F.; Yin, B.; Tlili, C.; Wang, D.; Qiu, S.; Li, Q. High-efficiency dispersion and sorting of single-walled carbon nanotubes via non-covalent interactions. J. Mater. Chem. C 2017, 5, 11339–11368. [Google Scholar] [CrossRef]
- Arto, I.; Capellán-Pérez, I.; Lago, R.; Bueno, G.; Bermejo, R. The energy requirements of a developed world. Energy Sustain. Dev. 2016, 33, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, T.; Abbasi, S.A. Decarbonization of fossil fuels as a strategy to control global warming. Renew. Sust. Energ. Rev. 2011, 15, 1828–1834. [Google Scholar] [CrossRef]
- Cheong, I.P.-A.; Johari, M.; Said, H.; Treagust, D.F. What do you know about alternative energy? Development and use of a diagnostic instrument for upper secondary school science. Int. J. Sci. Educ. 2015, 37, 210–236. [Google Scholar] [CrossRef]
- Herzog, A.V.; Lipman, T.E.; Kammen, D.M. Renewable Energy Sources. Encyclopedia of Life Support. Systems (EOLSS). Forerunner Volume-‘Perspectives and Overview of Life Support. Systems and Sustainable Development 2001. Available online: http://www.eolss.com (accessed on 20 June 2018).
- Capasso, A.; Salamandra, L.; Chou, A.; Di Carlo, A.; Motta, N. Multi-wall carbon nanotube coating of fluorine-doped tin oxide as an electrode surface modifier for polymer solar cells. Sol. Energy Mater. Sol. 2014, 122, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G. Transparent conductors as solar energy materials: A panoramic review. Sol. Energy Mater. Sol. 2007, 91, 1529–1598. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Yimao, W.; Chris, S.; James, B.; Mark, H.; Thomas, A.; Di, Y.; Jun, P.; Yiliang, W.; Jie, C.; Ali, J.; et al. Conductive and stable magnesium oxide electron-selective contacts for efficient silicon solar cells. Adv. Energy Mater. 2017, 7, 1601863. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Huang, Y.; Wei, J.; Liu, F.; Shao, Z.; Hu, L.; Chen, S.; Yang, S.; Tang, J.; et al. Efficient inorganic solid solar cells composed of perovskite and PbS quantum dots. Nanoscale 2015, 7, 9902–9907. [Google Scholar] [CrossRef]
- Shi, S.; Li, Y.; Li, X.; Wang, H. Advancements in all-solid-state hybrid solar cells based on organometal halide perovskites. Mater. Horiz. 2015, 2, 378–405. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, A.; Green, M.A.; Ferrazza, F. 19.8% efficient “honeycomb” textured multicrystalline and 24.4% monocrystalline silicon solar cells. Appl. Phys. Lett. 1998, 73, 1991–1993. [Google Scholar] [CrossRef]
- Saga, T. Advances in crystalline silicon solar cell technology for industrial mass production. Npg Asia Mater. 2010, 2, 96. [Google Scholar] [CrossRef] [Green Version]
- National Renewable Energy Laboratory (NREL). Research Cell Record Efficiency Chart. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 20 June 2018).
- Deibel, C.; Dyakonov, V. Polymer? fullerene bulk heterojunction solar cells. Rep. Prog. Phys. 2010, 73, 096401. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Ohkita, H.; Benten, H.; Ito, S. Charge generation and recombination dynamics in poly (3-hexylthiophene)/fullerene blend films with different regioregularities and morphologies. J. Am. Chem. Soc. 2010, 132, 6154–6164. [Google Scholar] [CrossRef] [PubMed]
- Movla, H.; Rafi, A.M.; Rafi, N.M. A model for studying the performance of P3HT: PCBM organic bulk heterojunction solar cells. J. Am. Chem. Soc. 2015, 126, 1429–1432. [Google Scholar]
- Li, M.; Gao, K.; Wan, X.; Zhang, Q.; Kan, B.; Xia, R.; Liu, F.; Yang, X.; Feng, H.; Ni, W.; et al. Solution-processed organic tandem solar cells with power conversion efficiencies >12%. Nat. Photonics 2016, 11, 85–90. [Google Scholar] [CrossRef]
- Babu, V.J.; Vempati, S.; Sundarrajan, S.; Sireesha, M.; Ramakrishna, S. Effective nanostructred morphologies for efficient hybrid solar cells. J. Sol. Energy 2014, 106, 1–22. [Google Scholar] [CrossRef]
- Chavhan, S.D.; Abellon, R.D.; van Breemen, A.J.J.M.; Koetse, M.M.; Sweelssen, J.; Savenije, T.J. Sensitization of p-type NiO using n-type conducting polymers. J. Phys. Chem. C 2010, 114, 19496–19502. [Google Scholar] [CrossRef]
- Ren, S.; Chang, L.-Y.; Lim, S.-K.; Zhao, J.; Smith, M.; Zhao, N.; Bulovic, V.; Bawendi, M.; Gradecak, S. Inorganic–organic hybrid solar cell: Bridging quantum dots to conjugated polymer nanowires. Nano lett. 2011, 11, 3998–4002. [Google Scholar] [CrossRef]
- Takagahara, T.; Takeda, K. Theory of the quantum confinement effect on excitons in quantum dots of indirect-gap materials. Phys. Rev. B 1992, 46, 15578. [Google Scholar] [CrossRef]
- Arici, E.; Meissner, D.; Schäffler, F.; Sariciftci, N.S. Core/shell nanomaterials in photovoltaics. Int J. photoenergy 2003, 5, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Qiao, Q. Conjugated polymer–inorganic semiconductor hybrid solar cells. Energy Environ. Sci. 2011, 4, 2700–2720. [Google Scholar] [CrossRef]
- Zhou, Y.; Eck, M.; Krüger, M. Bulk-heterojunction hybrid solar cells based on colloidal nanocrystals and conjugated polymers. Energy Environ. Sci. 2010, 3, 1851–1864. [Google Scholar] [CrossRef]
- Saunders, B.R. Hybrid polymer/nanoparticle solar cells: Preparation, principles and challenges. J. Colloid Interface Sci. 2012, 369, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhang, M.; Wang, X.; Yang, F.; Meng, X. Recent progress in organic–inorganic hybrid solar cells. J. Mater. Chem. A 2013, 1, 8694–8709. [Google Scholar] [CrossRef]
- Kim, J.; Ishihara, M.; Koga, Y.; Tsugawa, K.; Hasegawa, M.; Iijima, S. Low-temperature synthesis of large-area graphene-based transparent conductive films using surface wave plasma chemical vapor deposition. Appl. Phys. Lett. 2011, 98, 091502. [Google Scholar] [CrossRef]
- Fraga Domínguez, I.; Distler, A.; Lüer, L. Stability of organic solar cells: The influence of nanostructured carbon materials. Adv. Energy Mater. 2017, 7, 1601320. [Google Scholar] [CrossRef]
- Kavitha, M.K.; Jaiswal, M. Graphene: A review of optical properties and photonic applications. Asian J. Phys. 2016, 25, 809–831. [Google Scholar]
- Keru, G.; Ndungu, P.G.; Nyamori, V.O. A review on carbon nanotube/polymer composites for organic solar cells. Int. J. Energy Res. 2014, 38, 1635–1653. [Google Scholar] [CrossRef]
- Dutta, S.; Kim, J.; Ide, Y.; Ho Kim, J.; Hossain, M.S.A.; Bando, Y.; Yamauchi, Y.; Wu, K.C.W. 3D network of cellulose-based energy storage devices and related emerging applications. Mater. Horiz. 2017, 4, 522–545. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Shao, R.; Liang, J.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Biomass-derived mesopore-dominant porous carbons with large specific surface area and high defect density as high performance electrode materials for Li-ion batteries and supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Wu, H.; Mou, J.; Zhou, L.; Zheng, Q.; Jiang, N.; Lin, D. Cloud cap-like, hierarchically porous carbon derived from mushroom as an excellent host cathode for high performance lithium-sulfur batteries. Electrochim. Acta 2016, 212, 1021–1030. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Y.; Lu, Y.; Hu, Y.-S.; Li, J. A high-performance sodium-ion battery enhanced by macadamia shell derived hard carbon anode. Nano Energy 2017, 39, 489–498. [Google Scholar] [CrossRef]
- Niu, J.; Liang, J.; Shao, R.; Liu, M.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Tremella-like N,O-codoped hierarchically porous carbon nanosheets as high-performance anode materials for high energy and ultrafast Na-ion capacitors. Nano Energy 2017, 41, 285–292. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Xu, G.; Zhang, X.; Wang, S.; Li, C.; Wang, G. Hierarchical porous carbon derived from soybean hulls as a cathode matrix for lithium-sulfur batteries. J. Alloys Compd. 2017, 695, 2246–2252. [Google Scholar] [CrossRef]
- Półrolniczak, P.; Nowicki, P.; Wasiński, K.; Pietrzak, R.; Walkowiak, M. Biomass-derived hierarchical carbon as sulfur cathode stabilizing agent for lithium-sulfur batteries. Solid State Ion. 2016, 297, 59–63. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Wang, J.; Nie, P.; Ding, B.; Dong, S.; Hao, X.; Dou, H.; Zhang, X. Biomass derived carbon for energy storage devices. J. Mater. Chem. A 2017, 5, 2411–2428. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Y.; Chen, Y.; Guo, H. Activated biomass carbon made from bamboo as electrode material for supercapacitors. Mater. Res. Bull. 2018, 102, 391–398. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q. Activated carbon derived from harmful aquatic plant for high stable supercapacitors. Chem. Phys. Lett. 2018, 691, 238–242. [Google Scholar] [CrossRef]
- Virtanen, J.; Pammo, A.; Keskinen, J.; Sarlin, E.; Tuukkanen, S. Pyrolysed cellulose nanofibrils and dandelion pappus in supercapacitor application. Cellulose 2017, 24, 3387–3397. [Google Scholar] [CrossRef]
- Ruan, C.-Q.; Wang, Z.; Lindh, J.; Strømme, M. Carbonized cellulose beads for efficient capacitive energy storage. Cellulose 2018, 25, 3545–3556. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, A.A.; Chen, C.; Zhu, Z. Low-cost, high-performance supercapacitor based on activated carbon electrode materials derived from baobab fruit shells. J. Colloid Interface Sci. 2019, 538, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, D.; Luo, C.; Fu, Q.; Pan, C. Highly porous graphitic biomass carbon as advanced electrode materials for supercapacitors. Green Chem. 2017, 19, 4132–4140. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Dong, H.; Hu, H.; Zhao, X.; Cai, Y.; Xiao, Y.; Liu, Y.; Zheng, M. Super-hierarchical porous carbons derived from mixed biomass wastes by a stepwise removal strategy for high-performance supercapacitors. J. Power Sources 2018, 377, 151–160. [Google Scholar] [CrossRef]
- Shen, F.; Su, J.; Zhu, L.; Qi, X.; Zhang, X. Comprehensive utilization of dairy manure to produce glucose and hierarchical porous carbon for supercapacitors. Cellulose 2017, 24, 2571–2579. [Google Scholar] [CrossRef]
- Su, X.-L.; Chen, J.-R.; Zheng, G.-P.; Yang, J.-H.; Guan, X.-X.; Liu, P.; Zheng, X.-C. Three-dimensional porous activated carbon derived from loofah sponge biomass for supercapacitor applications. Appl. Surf. Sci. 2018, 436, 327–336. [Google Scholar] [CrossRef]
- Sun, J.; Niu, J.; Liu, M.; Ji, J.; Dou, M.; Wang, F. Biomass-derived nitrogen-doped porous carbons with tailored hierarchical porosity and high specific surface area for high energy and power density supercapacitors. Appl. Surf. Sci. 2018, 427, 807–813. [Google Scholar] [CrossRef]
- Sun, F.; Wang, L.; Peng, Y.; Gao, J.; Pi, X.; Qu, Z.; Zhao, G.; Qin, Y. Converting biomass waste into microporous carbon with simultaneously high surface area and carbon purity as advanced electrochemical energy storage materials. Appl. Surf. Sci. 2018, 436, 486–494. [Google Scholar] [CrossRef]
- Song, P.; Shen, X.; He, X.; Feng, K.; Kong, L.; Ji, Z.; Zhai, L.; Zhu, G.; Zhang, D. Cellulose-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitors. Cellulose 2018, 26, 1195–1208. [Google Scholar] [CrossRef]
- Mugadza, K.; Ndungu, P.G.; Stark, A.; Nyamori, V.O. Ionic liquids and cellulose: Innovative feedstock for synthesis of carbon nanostructured material. Mater. Chem. Phys. 2019, 234, 201–209. [Google Scholar] [CrossRef]
- Mugadza, K.; Ndungu, P.G.; Stark, A.; Nyamori, V.O. Conversion of residue biomass into value added carbon materials: Utilisation of sugarcane bagasse and ionic liquids. J. Mater. Sci. 2019, 54, 12476–12487. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugadza, K.; Stark, A.; Ndungu, P.G.; Nyamori, V.O. Synthesis of Carbon Nanomaterials from Biomass Utilizing Ionic Liquids for Potential Application in Solar Energy Conversion and Storage. Materials 2020, 13, 3945. https://doi.org/10.3390/ma13183945
Mugadza K, Stark A, Ndungu PG, Nyamori VO. Synthesis of Carbon Nanomaterials from Biomass Utilizing Ionic Liquids for Potential Application in Solar Energy Conversion and Storage. Materials. 2020; 13(18):3945. https://doi.org/10.3390/ma13183945
Chicago/Turabian StyleMugadza, Kudzai, Annegret Stark, Patrick G. Ndungu, and Vincent O. Nyamori. 2020. "Synthesis of Carbon Nanomaterials from Biomass Utilizing Ionic Liquids for Potential Application in Solar Energy Conversion and Storage" Materials 13, no. 18: 3945. https://doi.org/10.3390/ma13183945
APA StyleMugadza, K., Stark, A., Ndungu, P. G., & Nyamori, V. O. (2020). Synthesis of Carbon Nanomaterials from Biomass Utilizing Ionic Liquids for Potential Application in Solar Energy Conversion and Storage. Materials, 13(18), 3945. https://doi.org/10.3390/ma13183945