Soldering of 7075 Aluminum Alloy Using Ni-P and Cu-Cr Electrodeposited Interlayers
Abstract
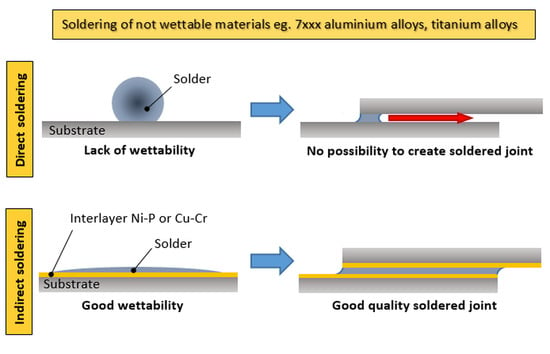
:1. Introduction
2. Materials and Methodology
3. Results and Discussion
3.1. Electrodeposition of Ni-P and Cu-Cr Coatings
- 8–12 g/dm3 of chromium metal in form of chromium chloride III;
- 12–15 g/dm3 of copper metal in form of copper chloride II;
- 80–120 g/dm3 of ammonium chloride as the conducting salt;
- pH of the solution within 3.8 to 4.5;
- process temperature within 55 to 65 °C;
- cathodic current density from 1.5 to 3.0 A/dm2;
- process time from 40 to 60 min.
- 15–18 g/dm3 of nickel metal in form of nickel sulphate II;
- 100–150 g/dm3 of phosphorus in form of nitrilotris(methylene)phosphonic acid;
- 100 g/dm3 of citric acid;
- pH of the solution within 1.5 to 2.5;
- process temperature within 45 to 55 °C;
- cathodic current density from 1.0 to 2.5 A/dm2;
- process time from 15 to 25 min.
3.2. Evaluation of Applied Coatings
3.3. Wettability Test
3.4. Metallographic Evaluation of Soldered Joints
3.5. Tests of Mechanical Properties of Soldered Joints
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simões, S. Diffusion bonding and brazing of advanced materials. Metals 2018, 8, 959. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, P.B.; Irving, B. Welding aluminum: It’s not as difficult as it sounds. Weld. J. 1992, 71, 45–50. [Google Scholar]
- Çevik, B. Gas tungsten arc welding of 7075 aluminum alloy: Microstructure properties, impact strength, and weld defects. Mater. Res. Express 2018, 5. [Google Scholar] [CrossRef]
- EN 485-2:2018 Aluminium and Aluminium Alloys—Sheet, Strip and Plate—Part 2: Mechanical Properties, Standard; European Committee for Standardization: Brussels, Belgium, December 2018.
- 7000 Series Aluminum Alloy. Available online: http://asm.matweb.com/search/SpecificMaterial.asp?bassnum=MA7075T6 (accessed on 14 July 2020).
- Metals Handbook, Vol.2-Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Cleveland, OH, USA, 1990.
- Lee, S.-Y.; Lee, J.-H.; Lee, Y.-S. Characterization of Al 7075 alloys after cold working and heating in the semi-solid temperature range. J. Mater. Process. Technol. 2011, 111, 42–47. [Google Scholar] [CrossRef]
- Kandil, H.M.; Salama, S.F.; Naggar, A.A. Mechanical and natural aging pretreatment of age hardenable 7075 aluminum alloy. J. Eng. Appl. Sci. 1999, 46, 46–65. [Google Scholar]
- Cheng, L.; Northwood, D.O.; Bhole, S.D. Tensile fracture behavior in CO2 laser beam welds of 7075-T6 aluminum alloy. Mater. Des. 2004, 25, 573–577. [Google Scholar] [CrossRef]
- Hirsch, J. Automotive trends in aluminium—The European perspective. Mater. Forum 2004, 28, 15–23. [Google Scholar]
- Kuczmaszewski, J.; Zalewski, K. Machining of Aluminum and Magnesium Alloys; Lublin University of Technology: Lublin, Poland, 2015. [Google Scholar]
- Pfeifer, T.; Stano, S. Modern methods of weld-brazing in the aspect of quality and properties of joints. Weld. Technol. Rev. 2016, 88, 95–102. [Google Scholar]
- Wojdat, T.; Kustroń, P.; Jaśkiewicz, K.; Zwierzchowski, M.; Margielewska, A. Numerical modelling of welding of car body sheets made of selected aluminium alloys. Arch. Metall. Mater. 2019, 64, 1403–1409. [Google Scholar] [CrossRef]
- Mirski, Z.; Wojdat, T.; Margielewska, A. Braze-welding in joining dissimilar materials. Inst. Weld. Bull. Gliw. 2018, 62, 26–32. (In Polish) [Google Scholar]
- Elrefaey, A. Effectiveness of cold metal transfer process for welding 7075 aluminium alloys. Sci. Technol. Weld. Join. 2015, 20, 280–285. [Google Scholar] [CrossRef]
- Gandhia, C.; Dixita, N.; Arankea, O.; Arivarasua, M.; Shanmugamb, N.S.; Manikandana, M.; Arivazhagan, N. Characterization of AA7075 Weldment using CMT Process. Mater. Today Proc. 2018, 5, 24024–24032. [Google Scholar] [CrossRef]
- EN ISO 18273:2016 Welding Consumables—Wire Electrodes, Wires and Rods for Welding of Aluminium and Aluminium Alloys—Classification, Standard; European Committee for Standardization: Brussels, Belgium, May 2016.
- Mirski, Z.; Pabian, J. Modern trends in production of brazed heat exchangers for automotive industry. Weld. Technol. Rev. 2017, 8, 5–12. (In Polish) [Google Scholar]
- Mirski, Z.; Wojdat, T.; Stachowicz, M. Soldering of aluminium with copper and steel using intermediate layer Zn–Ni. Arch. Civ. Mech. Eng. 2015, 15, 903–910. [Google Scholar] [CrossRef]
- Song, X.G.; Cao, J.; Feng, J.C.; Chai, J.H.; Hu, S.P.; Tang, D.Y. A novel metallization process for soldering graphite to copper at low temperature. J. Alloys Compd. 2017, 696, 1199–1204. [Google Scholar] [CrossRef]
- Wojdat, T.; Winnicki, M.; Łamasz, S.; Żuk, A. Application of interlayers in the soldering process of graphite composite to aluminium alloy 6060. Arch. Civ. Mech. Eng. 2019, 1, 91–99. [Google Scholar] [CrossRef]
- Walker, C.A.; Hodges, V.C. Comparing metal-ceramic brazing methods, the advantages and disadvantages of the various methods for joining metals to ceramics are outlined. Weld. J. 2008, 6, 43–50. [Google Scholar]
- Ciepacz, I.; Mirski, Z.; Wojdat, T.; Chęcmanowski, J.; Granat, K. Bath for the Galvanic Deposition of the Cu-Cr Alloy coaTing and the Method of Obtaining Cu-Cr Alloy Coatings on Conductive and Difficult to Bond Substrates. Patent Application No. P 427185, 2018. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.427185 (accessed on 18 July 2020).
- Ciepacz, I.; Mirski, Z.; Wojdat, T.; Chęcmanowski, J.; Porażka, Ł. Bath for the Galvanic Deposition of the Ni-P Alloy Coating and the Method of Obtaining Ni-P Alloy Coatings on Conductive and Difficult to Bond Substrates. Patent Application No. P 427186, 2018. Available online: https://ewyszukiwarka.pue.uprp.gov.pl/search/pwp-details/P.427186 (accessed on 18 July 2020).
- Harris Calorific International. Production Brazing Consumables and Equipment. Catalog, 2017. Available online: https://www.harrisproductsgroup.com/en/Catalogs.aspx (accessed on 16 July 2020).
- EN ISO 9453:2014 Soft Solder Alloys. Chemical Compositions and Forms, Standard; European Committee for Standardization: Brussels, Belgium, August 2014.
- EN 573-3:2014 Aluminium and Aluminium Alloys—Chemical Composition and Form of Wrought Products—Part 3: Chemical Composition and Form of Products, Standard; European Committee for Standardization: Brussels, Belgium, 2014; Available online: https://metcenter.ru/f/en_573-3.pdf (accessed on 15 July 2020).
- Documentation of Krűss Goniometer, Hamburg, Technical Note, 2009. Available online: https://www.kruss-scientific.com/fileadmin/user_upload/website/literature/kruss-tn315-en.pdf (accessed on 18 July 2020).
- Rogowska, R. Surface free energy of thin-layer coatings deposited by means of the arc-vacuum method. Maint. Probl. 2006, 2, 193–203. [Google Scholar]
- Pourboix, M. Lectures on Electrochemical Corrosion; PWN: Warsaw, Poland, 1978. [Google Scholar]
- Zimniak, Z.; Mirski, Z.; Ciepacz, I.; Granat, K.; Wojdat, T. The influence of cold plasma-surface modification on the grip of the electroplating finish to graphite composite and for its usefulness in soldering processes. Weld. Technol. Rev. 2018, 2, 22–25. [Google Scholar] [CrossRef]
- Hartmann, H.; Hofmann, W.; Oiler, D.M. Contribution to the knowledge of Zinc-Chromium System. Abhandl. Braunschweig. Wiss. Ges 1955, 7, 100–106. [Google Scholar]
- EN ISO 2819: 2018 Metallic Coatings on Metallic Substrates—Electrodeposited and Chemically Deposited Coatings—Review of Methods Available for Testing Adhesion, Standard; European Committee for Standardization: Brussels, Belgium, 2018.
- Eustathopoulos, N.; Nicholas, M.; Drevet, B. Wettability at High Temperatures; Pergamon Materials Series; Pergamon: Oxford, UK, 1999; Volume 3. [Google Scholar]
- Schwartz, M.M. Brazing, Second Edition, the Materials Informations Society; ASM International: Cleveland, OH, USA, 2003. [Google Scholar]
- Roberts, P. Industrial Brazing Practice, 2nd ed.; CRC Press, Taylor and Francis Group: Abingdon, VA, USA, 2013. [Google Scholar]
- Massalski, T.B. Binary Alloys Phase Diagrams; ASM International: Cleveland, OH, USA, 1992; Volume 3. [Google Scholar]
- Wojdat, T.; Winnicki, M.; Rutkowska-Gorczyca, M.; Krupiński, S.; Kubica, K. Soldering aluminium to copper with the use of interlayers deposited by cold spraying. Arch. Civ. Mech. Eng. 2016, 16, 835–844. [Google Scholar] [CrossRef]
- EN ISO 6507: 2018-05 Metallic Materials—Vickers Hardness Test—Part 1: Test Method, Standard; European Committee for Standardization: Brussels, Belgium, 2018.
- Ahmadkhaniha, D.; Pinate, S.; Leisner, P.; Zanella, C. Electrodeposition of Ni high P composite coatings containing nano and submicro ceramic particles. In Proceedings of the EUROCORR 2017, 20th International Corrosion Congress & Process Safety Congress Annual Congress of the European Federation of Corrosion, Prague, Czech Republic, 3–7 September 2017; pp. 721–729. [Google Scholar]
- Riyadi, T.W.; Sarjito, M.; Riswan, R.A. Mechanical properties of Cr-Cu coatings produced by electroplating. In Proceedings of the AIP Conference Proceedings, Surakarta, Indonesia, 7–8 December 2016; Volume 1855. [Google Scholar] [CrossRef] [Green Version]










| Chemical Composition, wt % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
| Base Metal 7075 alloy | Max. 0.40 | Max. 0.50 | 1.20–2.00 | Max. 0.30 | 2.10–2.90 | 0.18–0.28 | 5.10–6.10 | Max. 0.20 | Rem. |
| SP Analysis * | 0.12 | 0.07 | 1.80 | 0.09 | 2.88 | 0.19 | 6.04 | 0.06 | Rem. |
| Element | Cu | Fe | In | Bi | Pb | Ag | Ni | Other | Sn |
| S-Sn97Cu3 | 2.50–3.50 | Max. 0.02 | Max. 0.10 | Max. 0.07 | Max. 0.10 | Max. 0.10 | Max. 0.01 | Max. 0.186 | Rem. |
| SP Analysis * | 2.65 | 0.01 | 0.08 | 0.04 | 0.05 | 0.09 | 0.01 | 0.18 | Rem. |
| Liquid | Surface Free Energy (SFE), γw [mJ/m2] | Dispersion Component, γwD [mJ/m2] | Polar Component, γwP [mJ/m2] |
|---|---|---|---|
| Distilled Water | 72.8 | 21.8 | 51.0 |
| Diiodomethane | 50.8 | 48.5 | 2.3 |
| Ethylene Glycol | 48.0 | 29.0 | 19.0 |
| Coating | Thickness, µm | Average Thickness, µm | Element Content, wt % | |||
|---|---|---|---|---|---|---|
| Cr | AV Cr | P | AV P | |||
| Cu-Cr | 12.9 | 12.3 (σ = 0.9) | 1.17 | 1.12 (σ = 0.09) | _ | _ |
| 13.0 | 1.17 | |||||
| 12.9 | 1.08 | |||||
| 11.9 | 1.19 | |||||
| 10.9 | 0.98 | |||||
| Ni-P | 12.7 | 12.1 (σ = 0.7) | _ | _ | 12.2 | 11.6 (σ = 0.5) |
| 12.8 | 11.2 | |||||
| 11.9 | 11.9 | |||||
| 11.0 | 10.9 | |||||
| 11.9 | 11.9 | |||||
| Substrate | Surface Free Wnergy (SFE), γw [mL/m2] | Dispersion Component, γwD [mJ/m2] | Polar Component, γwP [mJ/m2] | Contact Angle, ° | ||
|---|---|---|---|---|---|---|
| Distilled Water | Diiodomethane | Ethylene Glycol | ||||
| 7075 | 41.7 | 36.8 | 4.9 | 92.1 | 68.2 | 57.9 |
| Cu-Cr | 58.8 | 42.5 | 16.3 | 49.4 | 43.8 | 41.2 |
| Ni-P | 66.9 | 43.1 | 23.8 | 38.7 | 32.4 | 22.6 |
| No. | Lap Joint | Shear Force Ft [N] | Shear Strength Rt [MPa] | Average Shear Strength Rta [MPa] | Fracture Type | |
|---|---|---|---|---|---|---|
| Dimensions [mm × mm] | Area of Joint [mm2] | |||||
| Cu-Cr Interlayer | ||||||
| 1 | 24.0 × 9.8 | 235 | 8600 | 36.6 | 35.4 (σ = 1.2) | Cohesive |
| 2 | 24.0 × 10.0 | 240 | 8100 | 33.8 | ||
| 3 | 23.8 × 9.7 | 231 | 8500 | 36.3 | ||
| 4 | 23.9 × 9.7 | 232 | 8300 | 35.8 | ||
| 5 | 23.4 × 9.9 | 232 | 8250 | 34.5 | ||
| Ni-P Interlayer | ||||||
| 1 | 25.1 × 9.1 | 228 | 7550 | 33.1 | 33.2 (σ = 0.8) | Cohesive |
| 2 | 25.1 × 8.3 | 208 | 6700 | 32.2 | ||
| 3 | 25.0 × 8.5 | 212 | 7300 | 34.4 | ||
| 4 | 25.3 × 8.8 | 223 | 8200 | 32.8 | ||
| 5 | 25.2 × 8.6 | 218 | 7950 | 33.5 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirski, Z.; Ciepacz, I.; Wojdat, T. Soldering of 7075 Aluminum Alloy Using Ni-P and Cu-Cr Electrodeposited Interlayers. Materials 2020, 13, 4100. https://doi.org/10.3390/ma13184100
Mirski Z, Ciepacz I, Wojdat T. Soldering of 7075 Aluminum Alloy Using Ni-P and Cu-Cr Electrodeposited Interlayers. Materials. 2020; 13(18):4100. https://doi.org/10.3390/ma13184100
Chicago/Turabian StyleMirski, Zbigniew, Ireneusz Ciepacz, and Tomasz Wojdat. 2020. "Soldering of 7075 Aluminum Alloy Using Ni-P and Cu-Cr Electrodeposited Interlayers" Materials 13, no. 18: 4100. https://doi.org/10.3390/ma13184100
APA StyleMirski, Z., Ciepacz, I., & Wojdat, T. (2020). Soldering of 7075 Aluminum Alloy Using Ni-P and Cu-Cr Electrodeposited Interlayers. Materials, 13(18), 4100. https://doi.org/10.3390/ma13184100