The Impact of Interphase Precipitation on the Mechanical Behavior of Fire-Resistant Steels at an Elevated Temperature
Abstract
:1. Introduction
2. Materials and Methods
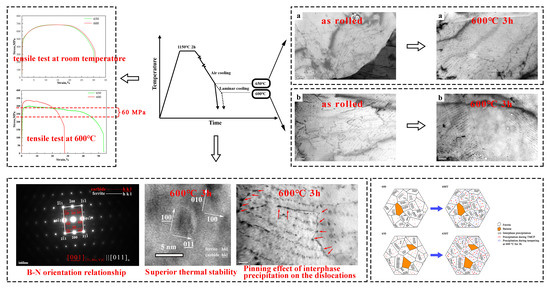
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gray, J.M.; Yeo, R.B.S. Columbium carbonitride precipitation in low-alloy steels with particular emphasis on precipitate-row formation. ASM Trans. Q. 1968, 61, 255–269. [Google Scholar]
- Funakawa, Y. Interphase Precipitation and Application to Practical Steels. Mater. Trans. 2019, 60, 2086–2095. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Shinbo, K.; Ohmura, T.; Suzuki, T.; Tsuzaki, K.; Miyamoto, G.; Furuhara, T. Randomization of Ferrite/austenite Orientation Relationship and Resultant Hardness Increment by Nitrogen Addition in Vanadium-microalloyed Low Carbon Steels Strengthened by Interphase Precipitation. ISIJ Int. 2018, 58, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.T.; Hodgson, P.D.; Bikmukhametov, I.; Miller, M.K.; Timokhina, I. Effects of hot-deformation on grain boundary precipitation and segregation in Ti-Mo microalloyed steels. Mater. Des. 2018, 141, 48–56. [Google Scholar] [CrossRef]
- Funakawa, Y.; Shiozaki, T.; Tomita, K.; Yamamoto, T.; Maeda, E. Development of high strength hot-rolled sheet steel consisting of ferrite and nanometer-sized carbides. ISIJ Int. 2004, 44, 1945–1951. [Google Scholar] [CrossRef]
- Yen, H.W.; Huang, C.Y.; Yang, J.R. Characterization of interphase-precipitated nanometer-sized carbides in a Ti–Mo-bearing steel. Scr. Mater. 2009, 61, 616–619. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, P.Y.; Huang, C.Y.; Yang, J.R. Interphase precipitation of nanometer-sized carbides in a titanium–molybdenum-bearing low-carbon steel. Acta Mater. 2011, 59, 6264–6274. [Google Scholar] [CrossRef]
- Mukherjee, S.; Timokhina, I.B.; Zhu, C.; Ringer, S.P.; Hodgson, P.D. Three-dimensional atom probe microscopy study of interphase precipitation and nanoclusters in thermomechanically treated titanium–molybdenum steels. Acta Mater. 2013, 61, 2521–2530. [Google Scholar] [CrossRef]
- Bikmukhametov, I.; Beladi, H.; Wang, J.T.; Hodgson, P.D.; Timokhina, I. The effect of strain on interphase precipitation characteristics in a Ti-Mo steel. Acta Mater. 2019, 170, 75–86. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Clark, S.J.; Cai, B.; Venero, D.A.; Yun, K.; Gorley, M.; Surrey, E.; McCartney, D.G.; Sridhar, S.; Lee, P.D. Small-angle neutron scattering reveals the effect of Mo on interphase nano-precipitation in Ti-Mo micro-alloyed steels. Scr. Mater. 2020, 174, 24–28. [Google Scholar] [CrossRef]
- Hashemi, S.G.; Eghbali, B. Analysis of the formation conditions and characteristics of interphase and random vanadium precipitation in a low-carbon steel during isothermal heat treatment. Int. J. Miner. Metall. Mater. 2018, 25, 339–349. [Google Scholar] [CrossRef]
- Gong, P.; Liu, X.G.; Rijkenberg, A.; Rainforth, W.M. The effect of molybdenum on interphase precipitation and microstructures in microalloyed steels containing titanium and vanadium. Acta Mater. 2018, 161, 374–387. [Google Scholar] [CrossRef] [Green Version]
- Bu, F.Z.; Wang, X.M.; Yang, S.W.; Shang, C.J.; Misra, R.D.K. Contribution of interphase precipitation on yield strength in thermomechanically simulated Ti-Nb and Ti-Nb-Mo microalloyed steels. Mater. Sci. Eng. A 2014, 620, 22–29. [Google Scholar] [CrossRef]
- Chen, J.; Lv, M.Y.; Tang, S.; Liu, Z.T.; Wang, G.D. Influence of cooling paths on microstructural characteristics and precipitation behaviors in a low carbon V–Ti microalloyed steel. Mater. Sci. Eng. A 2014, 594, 389–393. [Google Scholar] [CrossRef]
- Zhao, J.W.; Jiang, Z.Y. Thermomechanical processing of advanced high strength steels. Prog. Mater. Sci. 2018, 94, 174–242. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, S.F.; Chen, C.C.; Yang, J.R. Control of precipitation morphology in the novel HSLA steel. Mater. Sci. Eng. A 2015, 634, 123–133. [Google Scholar]
- Hutchinson, B. Microstructure development during cooling of hot rolled steels. Ironmak. Steelmak. 2001, 28, 145–151. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, C.Y.; Wang, T.Y.; Huang, C.Y.; Yang, J.R. Orientation relationship transition of nanometer sized interphase precipitated TiC carbides in Ti bearing steel. Mater. Sci. Technol. 2010, 26, 421–430. [Google Scholar] [CrossRef]
- Kamikawa, N.; Sato, K.; Miyamoto, G.; Murayama, M.; Sekido, N.; Tsuzakie, K.; Furuhara, T. Stress–strain behavior of ferrite and bainite with nano-precipitation in low carbon steels. Acta Mater. 2015, 83, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y. Origin of hydrogen trapping site in vanadium carbide precipitation strengthening steel. Acta Mater. 2018, 153, 193–204. [Google Scholar] [CrossRef]
- Sha, W.; Kirby, B.R.; Kelly, F.S. The behaviour of structural steels at elevated temperatures and the design of fire resistant steels. Mater. Trans. 2001, 42, 1913–1927. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Sun, X.J.; Li, Z.D.; Yong, Q.L. Precipitation and strengthening behaviors of MC type carbides in a Ti-V low carbon steel during tempering process. J. Iron Steel Res. 2015, 27, 50–54. [Google Scholar]
- Zhang, Z.; Sun, X.; Li, Z.; Wang, X.; Yong, Q.; Wang, G. Effect of nanometer-sized carbides and grain boundary density on performance of Fe-C-Mo-M(M = Nb, V or Ti) fire resistant steels. Chin. J. Mater. Res. 2015, 29, 269–276. [Google Scholar]
- Wan, R.; Sun, F.; Zhang, L.; Shan, A. Development and study of high-strength low-Mo fire-resistant steel. Mater. Des. 2012, 36, 227–232. [Google Scholar] [CrossRef]
- Pezzato, L.; Gennari, C.; Chukin, D.; Toldo, M.; Sella, F.; Toniolo, M.; Zambon, A.; Brunelli, K.; Dabalà, M. Study of the effect of multiple tempering on the impact toughness of forged S690 structural steel. Metals 2020, 10, 507. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Sun, X.; Yong, Q.; Li, Z.; Wang, Z.; Wang, G. Precipitation behavior of nanometer-sized carbides in Nb-Mo microalloyed high strengh steeland its strengthening mechanism. Acta Metall. Sin. 2016, 52, 410–418. [Google Scholar]
- GB/T 228-2002, Metallic Materials–Tensile Testing at Ambient Temperature; Standards Press of China: Beijing, China, 2002; (eqv ISO 6892:1998).
- GB/T 4338-2015, Metallic Materials–Tensile Testing–Part 2: Method of Test at Elevated Temperature; Standards Press of China: Beijing, China, 2015; (eqv ISO 6892-2: 2011, MOD).
- Bhadeshia, H.K.D.H. Diffusional Transformations: A Theory for the Formation of Superledges. Phys. Stat. Solidi A 1982, 69, 745–750. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yang, J.R.; Chen, C.C.; Chen, S.F. Microstructural characterization and strengthening behavior of nanometer sized carbides in Ti-Mo microalloyed steels during continuous cooling process. Mater. Charact. 2016, 114, 18–29. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.H.; Heo, Y.U.; Suh, D.W. Stability of (Ti, M) C (M = Nb, V, Mo and W) carbide in steels using first-principles calculations. Acta Mater. 2012, 60, 208–217. [Google Scholar] [CrossRef]
- Li, X.L.; Lei, C.S.; Deng, X.T.; Li, Y.M.; Tian, Y.; Wang, Z.D.; Wang, G.D. Carbide precipitation in ferrite in Nb-V-bearing low-carbon steel during isothermal quenching process. Acta Metall. Sin. Engl. Lett. 2017, 30, 1067–1079. [Google Scholar] [CrossRef] [Green Version]
- Pickering, F.B.; Gladman, T. Metallurgical Developments in Carbon Steels. J. Iron Steel Inst. Lond. 1963, 81, 10–29. [Google Scholar]
- Sha, W.; Kelly, F.S.; Guo, Z.X. Microstructure and properties of nippon fire-resistant steels. J. Mater. Eng. Perform. 1999, 8, 606–612. [Google Scholar] [CrossRef]
- Foreman, A.J.E.; Makin, M.J. Dislocation movement through random arrays of obstacles. Philos. Mag. 1966, 14, 911–924. [Google Scholar] [CrossRef]
- Fukuhara, M.; Sanpei, A. Elastic Moduli and Internal Friction of Low Carbon and Stainless Steels as a Function of Temperature. ISIJ Int. 1993, 33, 508–512. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E., III. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray Debye-Scherrer spectrum. Philos. Mag. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Kocks, U.F. On the spacing of dispersed obstacles. Acta Mater. 1966, 14, 1629–1631. [Google Scholar] [CrossRef]
- Yong, Q.L. Secondary Phases in Steel; Metallurgical Industry Press: Beijing, China, 2006; pp. 46–47. [Google Scholar]










| C | Mn | Si | Cr | Mo | Nb | Ti | V | Cu | Ni | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.06 | 1.30 | 0.18 | 0.40 | 0.24 | 0.03 | 0.13 | 0.10 | 0.56 | 0.32 | 0.012 | 0.008 |
| Temperature | Room Temperature | −40 °C | Tensile of 600 °C | ||||
|---|---|---|---|---|---|---|---|
| Mechanical Property | YS (MPa) | TS (MPa) | Yield Ratio | El (%) | CVN (J) | YS (MPa) | TS (MPa) |
| Steel LC650 | 476.8 | 686.2 | 0.69 | 30.9 | 280 | 228.4 | 303.1 |
| Steel LC600 | 494.9 | 677.7 | 0.73 | 31.4 | 252 | 288.4 | 337.3 |
| Process | d/nm | ρ/m−2 | ||
|---|---|---|---|---|
| Steel LC650 | 5.70 ± 0.73 | 0.0204 | 0.0016 | 9.99 × 1013 |
| Steel LC600 | 6.45 ± 0.69 | 0.0367 | 0.0032 | 5.07 × 1013 |
| Steel LC650+600T | 6.53 ± 0.41 | 0.0314 | 0.0027 | 6.00 × 1013 |
| Steel LC600+600T | 5.95 ± 0.41 | 0.0320 | 0.0025 | 3.92 × 1013 |
| Process | E (600 °C)/GPa | E (RT)/GPa | υ | G (600 °C)/GPa | G (RT)/GPa |
|---|---|---|---|---|---|
| Steel LC650 | 105.6 | 177.0 | 0.291 | 40.9 | 68.6 |
| Steel LC600 | 136.5 | 177.2 | 0.291 | 52.9 | 68.6 |
| Process | |||
|---|---|---|---|
| Steel LC650 | 203.2 | 138.7 | 246.0 |
| Steel LC600 | 145.0 | 183.2 | 233.6 |
| Steel LC650+600T | 94.0 | 100.8 | 137.8 |
| Steel LC600+600T | 98.2 | 133.1 | 165.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, J.; Li, J.; Fan, J.; Liu, P.; Misra, R.D.K.; Shang, C.; Wang, X. The Impact of Interphase Precipitation on the Mechanical Behavior of Fire-Resistant Steels at an Elevated Temperature. Materials 2020, 13, 4294. https://doi.org/10.3390/ma13194294
Cong J, Li J, Fan J, Liu P, Misra RDK, Shang C, Wang X. The Impact of Interphase Precipitation on the Mechanical Behavior of Fire-Resistant Steels at an Elevated Temperature. Materials. 2020; 13(19):4294. https://doi.org/10.3390/ma13194294
Chicago/Turabian StyleCong, Jinghua, Jiangwen Li, Jiajie Fan, Pengcheng Liu, Raja Devesh Kumar Misra, Chengjia Shang, and Xuemin Wang. 2020. "The Impact of Interphase Precipitation on the Mechanical Behavior of Fire-Resistant Steels at an Elevated Temperature" Materials 13, no. 19: 4294. https://doi.org/10.3390/ma13194294
APA StyleCong, J., Li, J., Fan, J., Liu, P., Misra, R. D. K., Shang, C., & Wang, X. (2020). The Impact of Interphase Precipitation on the Mechanical Behavior of Fire-Resistant Steels at an Elevated Temperature. Materials, 13(19), 4294. https://doi.org/10.3390/ma13194294