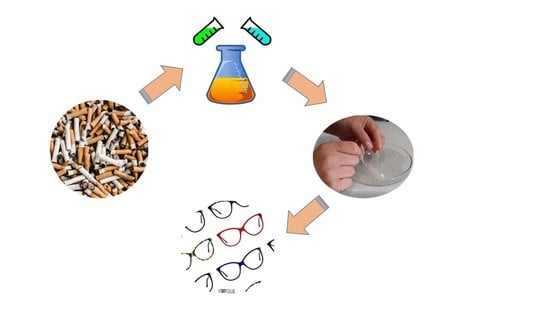
A Clean Process for Obtaining High-Quality Cellulose Acetate from Cigarette Butts
Abstract
:1. Introduction
2. Experimental
Materials and Methods
3. Results and Discussion
3.1. Morphological Characterization
3.2. Thermal Degradation Study
3.3. Characterization of Recovered CA by FTIR Spectroscopy
3.4. Reprocessing Waste Cigarette Butts into Usable Material
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Browne, C.L. The Design of Cigarettes; Hoechst Celanese: Irving, TX, USA, 1990; 119p. [Google Scholar]
- Du, W.; Tang, L.J.; Wen, J.H.; Zhong, K.J.; Jiang, J.H.; Wang, H.; Chen, B.; Yu, R.Q. Desorption corona beam ionisation (DCBI) mass spectrometry for in-situ analysis of adsorbed phenol in cigarette acetate fiber filter. Talanta 2015, 131, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, Y.; Umar, L. Preparation of creating active carbon from cigarette filter waste using microwave-induced KOH activation. J. Phys. Conf. Ser. 2017, 853, 012027. [Google Scholar] [CrossRef] [Green Version]
- Novotny, T.E.; Lum, K.; Smith, E.; Wang, V.; Barnes, R. Cigarettes butts and the case for an environmental policy on hazardous cigarette waste. Int. J. Environ. Res. Public Health 2009, 6, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Rowe, D.; Reid, M.J.; Thomas, K.V.; Galloway, T.S. Bioaccumulation and biological effects of cigarette litter in marine worms. Sci. Rep. Nat. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Kitajima, S.; Katahira, K. Waste on the roadside, ‘poi-sute’ waste: Its distribution and elution potential of pollutants into environment. Waste Manag. 2009, 29, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Kadir, A.A.; Sarani, N.A. Cigarette Butts Pollution and Environmental Impact—A Review. Appl. Mech. Mater. 2014, 773–774, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhang, N.; Qu, C.; Wu, X.; Zhang, J.; Zhang, X. Cigarette Butts and Their Application in Corrosion Inhibition for N80 Steel at 90 °C in a Hydrochloric Acid Solution. Ind. Eng. Chem. Res. 2010, 49, 3986–3991. [Google Scholar] [CrossRef]
- Mohajerani, A.; Kadir, A.A.; Larobina, L. A practical proposal for solving the world’s cigarette butt problem: Recycling in fired clay bricks. Waste Manag. 2016, 52, 228–244. [Google Scholar] [CrossRef]
- Kadir, A.A.; Mohajerani, A.; Roddick, F.; Buckeridge, J. Density, Strength, Thermal Conductivity and Leachate Characteristics of Light-Weight Fired Clay Bricks Incorporating Cigarette Butts. World Acad. Sci. Eng. Technol. 2009, 53, 135–1040. [Google Scholar]
- Ghosh, T.K.; Sadhukhan, S.; Rana, D.; Sarkar, G.; Das, C.; Chattopadhyay, S.; Chattopadhyay, D.; Chakraborty, M. Treatment of recycled cigarette butts (man-made pollutants) to prepare electrically conducting material. J. Indian Chem. Soc. 2017, 94, 863–870. [Google Scholar]
- Escobar, V.G.; Maderuelo-Sanz, R. Acoustical performance of samples prepared with cigarette butts. Appl. Acoust. 2017, 125, 166–172. [Google Scholar] [CrossRef]
- Ogundare, S.A.; Moodley, V.; van Zyl, W.E. Nanocrystalline cellulose isolated from discarded cigarette filters. Carbohydr. Polym. 2017, 175, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.B.D.H.; Duarte, M.A.B.; Garcez, L.R.; Rubim, J.C.; Gatti, T.H.; Suarez, P.A.Z. Process development for cigarette butts recycling into cellulose pulp. Waste Manag. 2017, 60, 140–150. [Google Scholar] [CrossRef]
- Abu-Danso, E.; Bagheri, A.; Bhatnagar, A. Facile functionalization of cellulose from discarded cigarette butts for the removal of diclofenac pharmaceutical from water. Carbohydr. Polym. 2019, 219, 46–55. [Google Scholar] [CrossRef]
- Huang, M.R.; Li, X.G. Thermal Degradation of Cellulose and Cellulose Esters. J. Appl. Polym. Sci. 1998, 68, 293–304. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Holter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Shafizadeh, F.; Bradbury, A.G.W. Thermal Degradation of Cellulose in Air and Nitrogen at Low Temperatures. J. Appl. Polym. Sci. 1979, 23, 1431–1442. [Google Scholar] [CrossRef]
- Maria da Conceição, C.L.; de Alencar, A.E.V.; Mazzeto, S.E.; de A Soares, S. The effect of additives on the thermal degradation of cellulose acetate. Polym. Degrad. Stab. 2003, 80, 149–155. [Google Scholar]
- Doyle, C.D. Kinetic Analysis of Thermogravimetric Data. J. Appl. Polym. Sci. 1961, 15, 285–292. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- ASTM. ASTM E698-05 Standard Test Method for Arrhenius Kinetic Constants for Thermally Unstable Materials; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Flynn, J.H. A General Differential Technique for the Determination of Parameters for d(α)/dt = f(α)A exp (−E/RT) Energy of Activation, Preexponential Factor and Order of Reaction (when Applicable). J. Therm. Anal. 1991, 37, 293–305. [Google Scholar] [CrossRef]
- Flynn, J.H. The Isoconversional Method for Determination of Energy of Activation at Constant Heating rates. Corrections for the Doyle Approximation. J. Therm. Anal. 1983, 27, 95–102. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Vincent, L.; Mija, A.; Guigo, N. Integral, differential and advanced isoconversional methods: Complex mechanisms and isothermal predicted conversion-time curves. Chemom. Intell. Lab. Syst. 2009, 96, 219–226. [Google Scholar] [CrossRef]
- Zsako, J. Kinetic analysis of thermogravimetric data. J. Phys. Chem. 1968, 72, 2406–2411. [Google Scholar] [CrossRef]
- Miura, K. New and simple method to estimate f(E) and k0(E) in the distributed activation energy model from three sets of experimental data. Energy Fuels 1995, 9, 302–307. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Carrier, M.; Meyer, E.L.; Knoetze, J.H.; Gorgens, J.F. Model fitting kinetic analysis and characterisation of the devolatilization of coal blends with corn and sugarcane residues. Thermochim. Acta 2012, 530, 95–106. [Google Scholar] [CrossRef]
- Micevska, T.; Warne, M.S.J.; Pablo, F.; Patra, R. Variation in, and causes of, toxicity of cigarette butts to a cladoceran and microtox. Arch. Environ. Contam. Toxicol. 2006, 50, 205–212. [Google Scholar] [CrossRef]
- Kazi, T.G.; Jalbani, N.; Arain, M.B.; Jamali, M.K.; Afridi, H.I.; Sarfraz, R.A.; Shah, A.Q. Toxic metals distribution in different components of Pakistani and imported cigarettes by electrothermal atomic absorption spectrometer. J. Hazard. Mater. 2009, 163, 302–307. [Google Scholar] [CrossRef]
- Kurmus, H.; Mohajerani, A. The toxicity and valorization options of cigarette butts. Waste Manag. 2020, 104, 104–118. [Google Scholar] [CrossRef]
- Kamide, K.; Saito, M. Thermal-analysis of cellulose-acetate solids with total degrees of substitution of 0.49, 1.75, 2.46, and 2.92. Polym. J. 1985, 17, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Rodrigues Filho, G.; Monteiro, D.S.; da Silva Meireles, C.; de Assunção, R.M.N.; Cerqueira, D.A.; Barud, H.S.; Ribeiro, S.J.; Messadeq, Y. Synthesis and characterization of cellulose acetate produced from recycled newspaper. Carbohydr. Polym. 2008, 73, 74–82. [Google Scholar] [CrossRef]












| Sample | Element | % w/w |
|---|---|---|
| CA unused | C | 49.3 |
| O | 50.5 | |
| Ti | 0.2 | |
| CA recovered | C | 50.7 |
| O | 49.1 | |
| Ti | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Fenzo, A.; Giordano, M.; Sansone, L. A Clean Process for Obtaining High-Quality Cellulose Acetate from Cigarette Butts. Materials 2020, 13, 4710. https://doi.org/10.3390/ma13214710
De Fenzo A, Giordano M, Sansone L. A Clean Process for Obtaining High-Quality Cellulose Acetate from Cigarette Butts. Materials. 2020; 13(21):4710. https://doi.org/10.3390/ma13214710
Chicago/Turabian StyleDe Fenzo, Anna, Michele Giordano, and Lucia Sansone. 2020. "A Clean Process for Obtaining High-Quality Cellulose Acetate from Cigarette Butts" Materials 13, no. 21: 4710. https://doi.org/10.3390/ma13214710
APA StyleDe Fenzo, A., Giordano, M., & Sansone, L. (2020). A Clean Process for Obtaining High-Quality Cellulose Acetate from Cigarette Butts. Materials, 13(21), 4710. https://doi.org/10.3390/ma13214710