Methane Combustion Using Pd Deposited on CeOx-MnOx/La-Al2O3 Pellistors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pd Deposited on CeOx-MnOx/La-Al2O3 Pellistors
2.1.1. CeOx-MnOx/La-Al2O3 Synthesis by Hydrothermal Method
2.1.2. Pd Deposition on CeOx-MnOx/La-Al2O3
2.1.3. Pellistor Preparation
2.2. Materials’ Characterization
2.3. Working Principle of the Pellistor in a Wheatstone Bridge
3. Results
3.1. Materials’ Characterization
3.1.1. X-ray Diffraction Analysis
3.1.2. Raman Spectroscopy
3.1.3. Scanning Electron Microscopy
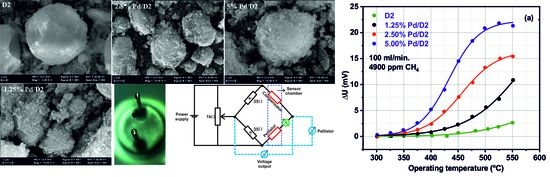
3.2. CH4 Catalytic Combustion Results
3.3. Modeling of the CH4 Catalytic Combustion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olah, G.A.; Goeppert, A.; Czaun, M.; Prakash, G.K.S. Sefl-Sufficient and Exclusive Oxigenation of Methane and Its Source Materials with Oxygen to Methanol via Metgas Using Oxidative Bi-reforming. J. Am. Chem. Soc. 2013, 135, 648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, X.; Wang, M.; Xie, Z.; Chen, H.; Shi, J. Highly active MnOx-CeO2 catalyst for diesel soot combustion. RSC Adv. 2017, 7, 3233–3239. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yu, F.; Zhu, M.; Wang, X.; Dan, J.; Zhang, J.; Cao, P.; Dai, B. Microspherical MnO2-CeO2-Al2O3 mixed oxide for monolithic honeycomb catalyst and application in selective catalytic reduction of NOx with NH3 at 50–150 °C. Chem. Eng. J. 2018, 346, 182–192. [Google Scholar] [CrossRef]
- Rubel, A.M.; Stencel, J.M. CH4 storage on compressed carbons. Fuel 2000, 79, 1095–1100. [Google Scholar] [CrossRef]
- Yang, N.-Z.; Guo, R.-T.; Tian, Y.; Pan, W.-G.; Chen, Q.-L.; Wang, Q.-S.; Lu, C.-Z.; Wang, S.-X. The enhanced performance of ceria by HF treatment for selective catalytic reduction of NO with NH3. Fuel 2016, 179, 305–311. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.-W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Li, S.; Yan, S.; Xia, Y.; Cui, B.; Pu, Y.; Ye, Y.; Wang, D.; Liu, Y.-Q.; Chen, B. Oxidative reactivity enhancement for soot combustion catalysts by co-doping silver and manganese in ceria. Appl. Catal. A Gen. 2019, 570, 299–307. [Google Scholar] [CrossRef]
- Müller, S.; Zimina, A.; Steininger, R.; Flessau, S.; Osswald, J.; Grunwaldt, J.D. High Stability of Rh Oxide-Based Thermoresistive Catalytic Combustion Sensors Proven by Operando X-ray Absorption Spectroscopy and X-ray Diffraction. ACS Sens. 2020, 5, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Kamieniak, J.; Randviir, E.P.; Banks, C.E. The latest developments in the analytical sensing of methane TrAC. Trends Anal. Chem. 2015, 73, 146–157. [Google Scholar] [CrossRef]
- Palmer, T.H. Gas Detecting Apparatus. U.S. Patent 3,233,233, 1 February 1966. [Google Scholar]
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Baiker, A. Axial variation of the oxidation state of Pt-Rh/Al2O3 during partial methane oxidation in a xed-bed reactor: An In Situ X-ray absorption spectroscopy study. Catal. Lett. 2005, 99, 5–12. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Basini, L.; Clausen, B.S. In Situ EXAFS study of Rh/Al2O3 catalysts for catalytic partial oxidation of methane. J. Catal. 2001, 200, 321–329. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Hobson, M.C.; Kennelly, T.; Waterman, E.M. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A Gen. 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K.; Urbano, F.J. Some aspects of hydrocarbon activation on platinum group metal combustion catalysis. Catal. Today 1996, 27, 243–248. [Google Scholar] [CrossRef]
- Lieske, H.; Volter, J. Pd Redispersion by Spreading of PdO in O2 Treated Pd/Al2O3. J. Phys. Chem. 1985, 89, 1841–1842. [Google Scholar] [CrossRef]
- Epling, W.S.; Hoflund, G.B. Catalytic oxidation of methane over ZrO2-Supported Pd catalysts. J. Catal. 1999, 182, 5–12. [Google Scholar] [CrossRef]
- Primavera, A.; Trovarelli, A.; Leitenburg, C. Reactivity and Characterization of Pd-containing ceria-zirconia catalysts for methane combustion. In Natural Gas Conversion V; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1998; Volume 119, pp. 87–92. ISBN 0-444-82967-9. [Google Scholar]
- Mukherjee, D.; Rao, B. G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B Environ. 2016, 197, 105–115. [Google Scholar] [CrossRef]
- Erdohelyi, A.; Cserenyi, J.; Solymosi, F. Activation of CH4 and Its Reaction with CO2 over Supported Rh Catalysts. J. Catal. 1993, 141, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tong, M.M.; Zhang, D.; Gao, Z. Improving the Performance of Catalytic Combustion Type Methane Gas Sensors Using Nanostructure Elements Doped with Rare Earth Cocatalysts. Sensors 2011, 11, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Genti, G.; Ciambelli, P.; Perathoner, S.; Russo, P. Environmental Catalysts: Trends and Outlook. ChemInform 2002, 75, 3–5. [Google Scholar] [CrossRef]
- Dey, S.; Kumar, P.V.V. The performance of highly active manganese oxide catalysts for ambient conditions carbon monoxide oxidation. Curr. Res. Green Sustain. Chem. 2020, 3, 100012. [Google Scholar] [CrossRef]
- Dardouri, R.; Gannouni, A.; Zina, S.M. Structural and oxidative properties of manganese incorporated mesostructured silica for methane oxidation. Adv. Mater. Sci. Eng. 2019, 6024876. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Xiumin, H.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W.; Li, Y. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Shi, L.; Chu, W.; Qu, F.; Luo, S. Low-temperature, catalytic combustion of methane over MnOx-CeO2 mixed oxide catalysts: Effect of preparation method. Catal. Lett. 2007, 113, 59–64. [Google Scholar] [CrossRef]
- Shi, L.; Chu, W.; Qu, F.; Hu, J.; Li, M. Catalytic performances for methane combustion of supported Mn-Ce mixed oxides. J. Rare Earth 2008, 266, 836–840. [Google Scholar] [CrossRef]
- Xiao, L.H.; Sun, K.P.; Xu, X.I.; Li, X.N. Low temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition-precipitation method. Catal. Commun. 2005, 6, 796–801. [Google Scholar] [CrossRef]
- Fiuk, M.M.; Adamski, A. Activity of MnOx-CeO2 catalysts in combustion of low concentrated methane. Catal. Today 2015, 257, 131–135. [Google Scholar] [CrossRef]
- Shimokawa, H.; Kurihara, Y.; Kusaba, H.; Einaga, H.; Teraoka, Y. Comparison of catalytic performance of Ag- and K-based catalysts for diesel soot combustion. Catal. Today 2012, 185, 99–103. [Google Scholar] [CrossRef]
- Neatu, S.; Trandafir, M.M.; Stanoiu, A.; Florea, O.G.; Simion, C.E.; Leonat, L.N.; Cobianu, C.; Gheorghe, M.; Florea, M.; Neatu, F. Bulk Versus Surface Modification of Alumina with Mn and Ce Based Oxides for CH4 Catalytic Combustion. Materials 2019, 12, 1771. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Sayari, A.; Adnot, A.; Larachi, F. Composition-activity effects of Mn-Ce-O composites on phenol catalytic wet oxidation. Appl. Catal. B Environ. 2001, 32, 195. [Google Scholar] [CrossRef]
- Liang, H.; Raitano, J.M.; He, G.; Akey, A.J.; Herman, I.P.; Zhang, L.; Chan, S.-W. Aqueous co-precipitation of Pd-doped cerium oxide nanoparticles: Chemistry, structure, and particle growth. J. Mater. Sci. 2012, 47, 299–307. [Google Scholar] [CrossRef]
- Ma, L.; Yuan, S.; Zhu, H.; Jiang, T.; Zhu, X.; Lu, C.; Li, X. Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane. Catalysts 2019, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Nitze, F.; Sharifi, T.; Barzegar, H.R.; Wagberg, T. Self-assembled palladium nanocrystals on helical carbon nanofibers as enhanced electrocatalysts for electro-oxidation of small molecules. J. Mater. Chem. 2012, 22, 8541–8548. [Google Scholar] [CrossRef]
- Baylet, A.; Marecot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In Situ Raman and in situ XRD analysis of PdO reduction and Pd0 oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx–CeO2–Al2O3 mixed oxides for soot oxidation: Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290. [Google Scholar] [CrossRef]
- Sato, T.; Komanoya, T. Selective oxidation of alcohols with molecular oxygen catalysed by Ru/MnOx/CeO2 under mild conditions. Catal. Commun. 2009, 10, 1095–1098. [Google Scholar] [CrossRef]
- Li, G.; Smith, R.L., Jr.; Inomata, H. Synthesis of Nanoscale Ce1-xFexO2 Solid Solutions via a Low-Temperature Approach. J. Am. Chem. Soc. 2001, 123, 11091–11092. [Google Scholar] [CrossRef]
- Venkataswamy, P.; Jampaiah, D.; Lin, F.; Alxneit, I.; Reddy, B.M. Structural properties of alumina supported Ce-Mn solid solutions and their markedly enhanced catalytic activity for CO oxidation. Appl. Surf. Sci. 2015, 349, 299–309. [Google Scholar] [CrossRef]
- Lee, J.H.; Trimm, D.L. Catalytic combustion of methane. Fuel Process. Technol. 1995, 42, 339–359. [Google Scholar] [CrossRef]
- Jones, T.A.; Walsh, P.T. Flammable Gas Detection—The role of the platinum metals. Platinum Met. Rev. 1988, 32, 50–60. [Google Scholar]
- Schierbaum, K.D.; Geiger, J.; Weimar, U.; Göpel, W. Specific palladium and platinum doping for SnO2-based thin film sensor arrays. Sens. Actuators B 1993, 13–14, 143–147. [Google Scholar] [CrossRef]
- Shlenkevitch, D.; Stolyarova, S.; Blank, T.; Brouk, I.; Nemirovsky, Y. Novel Miniature and selective Combustion-Type CMOS Gas Sensor for Gas-Mixture Analysis—Part 1: Emphasis on Chemical Aspects. Micromachines 2000, 11, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| EDX | Normalized Mass (%) | |||
|---|---|---|---|---|
| D2 | 1.25% Pd/D2 | 2.50% Pd/D2 | 5.00% Pd/D2 | |
| C | 13.80 | 13.61 | 13.01 | 11.10 |
| O | 33.92 | 53.12 | 49.63 | 46.17 |
| Al | 23.72 | 11.76 | 14.73 | 15.52 |
| Mn | 3.98 | 2.38 | 2.71 | 2.49 |
| La | 1.67 | 0.88 | 0.87 | 0.93 |
| Ce | 22.91 | 16.75 | 17.32 | 18.21 |
| Pd | - | 1.50 (1.38) 1 | 1.73 (2.21) 1 | 5.58 (5.61) 1 |
| Total | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florea, O.G.; Stănoiu, A.; Gheorghe, M.; Cobianu, C.; Neaţu, F.; Trandafir, M.M.; Neaţu, Ş.; Florea, M.; Simion, C.E. Methane Combustion Using Pd Deposited on CeOx-MnOx/La-Al2O3 Pellistors. Materials 2020, 13, 4888. https://doi.org/10.3390/ma13214888
Florea OG, Stănoiu A, Gheorghe M, Cobianu C, Neaţu F, Trandafir MM, Neaţu Ş, Florea M, Simion CE. Methane Combustion Using Pd Deposited on CeOx-MnOx/La-Al2O3 Pellistors. Materials. 2020; 13(21):4888. https://doi.org/10.3390/ma13214888
Chicago/Turabian StyleFlorea, Ovidiu G., Adelina Stănoiu, Marin Gheorghe, Cornel Cobianu, Florentina Neaţu, Mihaela M. Trandafir, Ştefan Neaţu, Mihaela Florea, and Cristian E. Simion. 2020. "Methane Combustion Using Pd Deposited on CeOx-MnOx/La-Al2O3 Pellistors" Materials 13, no. 21: 4888. https://doi.org/10.3390/ma13214888
APA StyleFlorea, O. G., Stănoiu, A., Gheorghe, M., Cobianu, C., Neaţu, F., Trandafir, M. M., Neaţu, Ş., Florea, M., & Simion, C. E. (2020). Methane Combustion Using Pd Deposited on CeOx-MnOx/La-Al2O3 Pellistors. Materials, 13(21), 4888. https://doi.org/10.3390/ma13214888