Multivariate Design of 3D Printed Immediate-Release Tablets with Liquid Crystal-Forming Drug—Itraconazole
Abstract
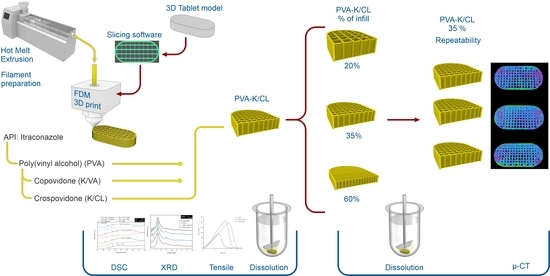
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Drug-Loaded Filaments
2.3. Evaluation of Filament Properties
2.4. Determination of Itraconazole Content in the Obtained Filament
2.5. Preparation of 3D Printed Tablets
2.6. Preparation of Tablets by Filament Compression (HME Tablets)
2.7. Preparation of Directly Compressed Tablets (DC Tablets)
2.8. Micro-Computed Tomography
2.9. Differential Scanning Calorimetry (DSC)
2.10. X-Ray Powder Diffraction (XRD)
2.11. Dissolution Studies
2.12. Solubility Study
3. Results
3.1. Evaluation of the Filaments
3.2. Thermal and Structural Properties of the Filaments and 3DP Tablets
3.3. Micro-Computed Tomography Studies of Tablets
3.4. Dissolution Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pandey, M.; Choudhury, H.; Fern, J.L.C.; Kee, A.T.K.; Kou, J.; Jing, J.L.J.; Her, H.C.; Yong, H.S.; Ming, H.C.; Bhattamisra, S.K.; et al. 3D printing for oral drug delivery: A new tool to customize drug delivery. Drug Deliv. Transl. Res. 2020, 10, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Isreb, A.; Forbes, R.T.; Dores, F.; Habashy, R.; Petit, J.-B.; Alhnan, M.A.; Oga, E.F. ‘Temporary Plasticiser’: A novel solution to fabricate 3D printed patient-centred cardiovascular ‘Polypill’ architectures. Eur. J. Pharm. Biopharm. 2019, 135, 94–103. [Google Scholar] [CrossRef]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Tagami, T.; Nagata, N.; Hayashi, N.; Ogawa, E.; Fukushige, K.; Sakai, N.; Ozeki, T. Defined drug release from 3D-printed composite tablets consisting of drug-loaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler. Int. J. Pharm. 2018, 543, 361–367. [Google Scholar] [CrossRef]
- Korte, C.; Quodbach, J. 3D-Printed Network Structures as Controlled-Release Drug Delivery Systems: Dose Adjustment, API Release Analysis and Prediction. AAPS PharmSciTech 2018, 19, 3333–3342. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Kurek, M.; Szafraniec-Szczęsny, J.; Czech, A.; Gawlak, K.; Knapik-Kowalczuk, J.; Leszczyński, B.; Wróbel, A.; Paluch, M.; Jachowicz, R. Speed it up, slow it down…An issue of bicalutamide release from 3D printed tablets. Eur. J. Pharm. Sci. 2020, 143, 105169. [Google Scholar] [CrossRef] [PubMed]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, J.J.; Awad, A.; Martorana, A.; Gaisford, S.; Stoyanov, E.; Basit, A.W.; Goyanes, A. 3D printed opioid medicines with alcohol-resistant and abuse-deterrent properties. Int. J. Pharm. 2020, 579, 119169. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef] [Green Version]
- Karakurt, I.; Aydoğdu, A.; Çıkrıkcı, S.; Orozco, J.; Lin, L. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: A controlled release study. Int. J. Pharm. 2020, 584, 119428. [Google Scholar] [CrossRef]
- Uddin, J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D printed microneedles for anticancer therapy of skin tumours. Mater. Sci. Eng. C 2020, 107, 110248. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Lin, X.; Yang, Q.; Yang, G. Printability of External and Internal Structures Based on Digital Light Processing 3D Printing Technique. Pharmaceutics 2020, 12, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206. [Google Scholar] [CrossRef]
- Sen, K.; Manchanda, A.; Mehta, T.; Ma, A.W.; Chaudhuri, B. Formulation design for inkjet-based 3D printed tablets. Int. J. Pharm. 2020, 584, 119430. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Fanous, M.; Gold, S.; Muller, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Simplification of fused deposition modeling 3D-printing paradigm: Feasibility of 1-step direct powder printing for immediate release dosage form production. Int. J. Pharm. 2020, 578, 119124. [Google Scholar] [CrossRef]
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards Printed Pediatric Medicines in Hospital Pharmacies: Comparison of 2D and 3D-Printed Orodispersible Warfarin Films with Conventional Oral Powders in Unit Dose Sachets. Pharmaceutics 2019, 11, 334. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Pan, H.; Fang, D.; Qiao, S.; Wang, S.; Pan, W. Fabrication of high drug loading levetiracetam tablets using semi-solid extrusion 3D printing. J. Drug Deliv. Sci. Technol. 2020, 57, 101683. [Google Scholar] [CrossRef]
- Karavasili, C.; Gkaragkounis, A.; Moschakis, T.; Ritzoulis, C.; Fatouros, D.G. Pediatric-friendly chocolate-based dosage forms for the oral administration of both hydrophilic and lipophilic drugs fabricated with extrusion-based 3D printing. Eur. J. Pharm. Sci. 2020, 147, 105291. [Google Scholar] [CrossRef]
- El Aita, I.; Breitkreutz, J.; Quodbach, J. Investigation of semi-solid formulations for 3D printing of drugs after prolonged storage to mimic real-life applications. Eur. J. Pharm. Sci. 2020, 146, 105266. [Google Scholar] [CrossRef]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Czech, A.; Szafraniec, J.; Gawlak, K.; Jachowicz, R. 3D printing of tablets containing amorphous aripiprazole by filaments co-extrusion. Eur. J. Pharm. Biopharm. 2018, 131, 44–47. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef]
- Nazir, A.; Jeng, J.-Y. A high-speed additive manufacturing approach for achieving high printing speed and accuracy. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2019, 234, 2741–2749. [Google Scholar] [CrossRef]
- Shaw, L.L.; Islam, M.; Li, J.; Li, L.; Ayub, S.M.I. High-Speed Additive Manufacturing Through High-Aspect-Ratio Nozzles. JOM 2018, 70, 284–291. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Speer, I.; Preis, M.; Breitkreutz, J. Novel Dissolution Method for Oral Film Preparations with Modified Release Properties. AAPS PharmSciTech 2018, 20, 7. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Öblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech 2019, 20, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Kapoor, Y.; Hermans, A.; Nofsinger, R.; Kesisoglou, F.; Gustafson, T.P.; Procopio, A. 3D printed capsules for quantitative regional absorption studies in the GI tract. Int. J. Pharm. 2018, 550, 418–428. [Google Scholar] [CrossRef]
- Melocchi, A.; Uboldi, M.; Parietti, F.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A.; Maroni, A.; Zema, L. Lego-Inspired Capsular Devices for the Development of Personalized Dietary Supplements: Proof of Concept With Multimodal Release of Caffeine. J. Pharm. Sci. 2020, 109, 1990–1999. [Google Scholar] [CrossRef]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Development of a dual extrusion printing technique for an acid- and thermo-labile drug. Eur. J. Pharm. Sci. 2018, 123, 191–198. [Google Scholar] [CrossRef]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef]
- Kimura, S.-I.; Ishikawa, T.; Iwao, Y.; Itai, S.; Kondo, H. Fabrication of Zero-Order Sustained-Release Floating Tablets via Fused Depositing Modeling 3D Printer. Chem. Pharm. Bull. 2019, 67, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Giri, B.R.; Song, E.S.; Kwon, J.; Lee, J.-H.; Park, J.-B.; Kim, D.S. Fabrication of Intragastric Floating, Controlled Release 3D Printed Theophylline Tablets Using Hot-Melt Extrusion and Fused Deposition Modeling. Pharmaceutics 2020, 12, 77. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Uboldi, M.; Inverardi, N.; Briatico-Vangosa, F.; Baldi, F.; Pandini, S.; Scalet, G.; Auricchio, F.; Cerea, M.; Foppoli, A.; et al. Expandable drug delivery system for gastric retention based on shape memory polymers: Development via 4D printing and extrusion. Int. J. Pharm. 2019, 571, 118700. [Google Scholar] [CrossRef]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef]
- Melocchi, A.; Parietti, F.; Maccagnan, S.; Ortenzi, M.A.; Antenucci, S.; Briatico-Vangosa, F.; Maroni, A.; Gazzaniga, A.; Zema, L. Industrial Development of a 3D-Printed Nutraceutical Delivery Platform in the Form of a Multicompartment HPC Capsule. AAPS PharmSciTech 2018, 19, 3343–3354. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, X.; Fang, R.; Xiao, Z.; Jin, Y. 3D printed mold-based capsaicin candy for the treatment of oral ulcer. Int. J. Pharm. 2019, 568, 118517. [Google Scholar] [CrossRef]
- He, S.; Feng, S.; Nag, A.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C. Recent Progress in 3D Printed Mold-Based Sensors. Sensors 2020, 20, 703. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Feng, S.; Mukhopadhyay, S.; Kosel, J.; Inglis, D. 3D printed mould-based graphite/PDMS sensor for low-force applications. Sens. Actuators A Phys. 2018, 280, 525–534. [Google Scholar] [CrossRef]
- Sarode, A.L.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H. Hot melt extrusion (HME) for amorphous solid dispersions: Predictive tools for processing and impact of drug-polymer interactions on supersaturation. Eur. J. Pharm. Sci. 2013, 48, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D printing of hydroxyapatite polymer-based composites for bone tissue engineering. J. Polym. Eng. 2017, 37, 741–746. [Google Scholar] [CrossRef]
- Solanki, N.G.; Lam, K.; Tahsin, M.; Gumaste, S.G.; Shah, A.V.; Serajuddin, A.T. Effects of Surfactants on Itraconazole-HPMCAS Solid Dispersion Prepared by Hot-Melt Extrusion I: Miscibility and Drug Release. J. Pharm. Sci. 2019, 108, 1453–1465. [Google Scholar] [CrossRef]
- Solanki, N.G.; Gumaste, S.G.; Shah, A.V.; Serajuddin, A.T. Effects of Surfactants on Itraconazole-Hydroxypropyl Methylcellulose Acetate Succinate Solid Dispersion Prepared by Hot Melt Extrusion. II: Rheological Analysis and Extrudability Testing. J. Pharm. Sci. 2019, 108, 3063–3073. [Google Scholar] [CrossRef]
- Jennotte, O.; Koch, N.; Lechanteur, A.; Evrard, B. Three-dimensional printing technology as a promising tool in bioavailability enhancement of poorly water-soluble molecules: A review. Int. J. Pharm. 2020, 580, 119200. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Potter, C.B.; Davis, M.T.; Iqbal, J.; Korde, S.; Pagire, S.; Paradkar, A.; Walker, G.M. Development of stability-enhanced ternary solid dispersions via combinations of HPMCP and Soluplus® processed by hot melt extrusion. Int. J. Pharm. 2017, 532, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Lee, W.Y.T.; Chow, A.H.L. Crystallization of Itraconazole Polymorphs from Melt. Cryst. Growth Des. 2016, 16, 3791–3801. [Google Scholar] [CrossRef]
- Heczko, D.; Kamińska, E.; Jurkiewicz, K.; Tarnacka, M.; Merkel, K.; Kamiński, K.; Paluch, M. The impact of various azole antifungals on the liquid crystalline ordering in itraconazole. J. Mol. Liq. 2020, 307, 112959. [Google Scholar] [CrossRef]
- Zhong, Y.; Jing, G.; Tian, B.; Huang, H.; Zhang, Y.; Gou, J.; Tang, X.; He, H.; Wang, Y. Supersaturation induced by Itraconazole/Soluplus® micelles provided high GI absorption in vivo. Asian J. Pharm. Sci. 2016, 11, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Bharati, A.; Frederiks, P.; Verkinderen, O.; Goderis, B.; Cardinaels, R.; Moldenaers, P.; Van Humbeeck, J.; Mooter, G.V.D. Effect of Compression on the Molecular Arrangement of Itraconazole-Soluplus Solid Dispersions: Induction of Liquid Crystals or Exacerbation of Phase Separation? Mol. Pharm. 2016, 13, 1879–1893. [Google Scholar] [CrossRef]
- Solanki, N.; Gupta, S.S.; Serajuddin, A.T. Rheological analysis of itraconazole-polymer mixtures to determine optimal melt extrusion temperature for development of amorphous solid dispersion. Eur. J. Pharm. Sci. 2018, 111, 482–491. [Google Scholar] [CrossRef]
- Miller, D.A.; DiNunzio, J.C.; Yang, W.; McGINITY, J.W.; Williams, R.O.; Williams, R.O. Targeted Intestinal Delivery of Supersaturated Itraconazole for Improved Oral Absorption. Pharm. Res. 2008, 25, 1450–1459. [Google Scholar] [CrossRef]
- Meng, F.; Meckel, J.; Zhang, F. Investigation of itraconazole ternary amorphous solid dispersions based on povidone and Carbopol. Eur. J. Pharm. Sci. 2017, 106, 413–421. [Google Scholar] [CrossRef]
- Parikh, T.; Serajuddin, A.T.M. Development of Fast-Dissolving Amorphous Solid Dispersion of Itraconazole by Melt Extrusion of its Mixture with Weak Organic Carboxylic Acid and Polymer. Pharm. Res. 2018, 35, 127. [Google Scholar] [CrossRef]
- Wlodarski, K.; Zhang, F.; Liu, T.; Sawicki, W.; Kipping, T. Synergistic Effect of Polyvinyl Alcohol and Copovidone in Itraconazole Amorphous Solid Dispersions. Pharm. Res. 2018, 35, 16. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Bauer, F.; Birk, G.; Lubda, D. Polyvinyl Alcohol in Hot Melt Extrusion to Improve the Solubility of Drugs. 2013. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/0/content/pdf/PS-PVA-HME-Improve-Solubility-03-2017_EN_MS.pdf (accessed on 27 October 2020).
- Malaquias, L.F.; Schulte, H.L.; Chaker, J.A.; Karan, K.; Durig, T.; Marreto, R.N.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Hot Melt Extrudates Formulated Using Design Space: One Simple Process for Both Palatability and Dissolution Rate Improvement. J. Pharm. Sci. 2018, 107, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Lang, B.; McGINITY, J.W.; Williams, R.O.; Williams, R.O. Dissolution Enhancement of Itraconazole by Hot-Melt Extrusion Alone and the Combination of Hot-Melt Extrusion and Rapid Freezing—Effect of Formulation and Processing Variables. Mol. Pharm. 2013, 11, 186–196. [Google Scholar] [CrossRef]
- Feng, D.; Peng, T.; Huang, Z.; Singh, V.; Shi, Y.; Wen, T.; Lu, M.; Quan, G.; Pan, X.; Wu, C. Polymer-Surfactant System Based Amorphous Solid Dispersion: Precipitation Inhibition and Bioavailability Enhancement of Itraconazole. Pharmaceutics 2018, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Solanki, N.G.; Kathawala, M.; Serajuddin, A.T. Effects of Surfactants on Itraconazole-Hydroxypropyl Methylcellulose Acetate Succinate Solid Dispersion Prepared by Hot Melt Extrusion III: Tableting of Extrudates and Drug Release From Tablets. J. Pharm. Sci. 2019, 108, 3859–3869. [Google Scholar] [CrossRef]
- Ponsar, H.; Wiedey, R.; Quodbach, J. Hot-Melt Extrusion Process Fluctuations and Their Impact on Critical Quality Attributes of Filaments and 3D-Printed Dosage Forms. Pharmaceutics 2020, 12, 511. [Google Scholar] [CrossRef]
- Knapik, J.; Jurkiewicz, K.; Kocot, A.; Paluch, M. Rheo-dielectric studies of the kinetics of shear-induced nematic alignment changes in itraconazole. J. Mol. Liq. 2020, 302, 112494. [Google Scholar] [CrossRef]
- Feuerbach, T.; Callau-Mendoza, S.; Thommes, M. Development of filaments for fused deposition modeling 3D printing with medical grade poly(lactic-co-glycolic acid) copolymers. Pharm. Dev. Technol. 2018, 24, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, E.; Forde, M.; Healy, A.V.; Gately, N.; Lyons, J.G.; McConville, C.; Major, I. Material Considerations for Fused-Filament Fabrication of Solid Dosage Forms. Pharmaceutics 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, A.; Abate, K.M.; Kumar, A.; Jeng, J.-Y. A state-of-the-art review on types, design, optimization, and additive manufacturing of cellular structures. Int. J. Adv. Manuf. Technol. 2019, 104, 3489–3510. [Google Scholar] [CrossRef]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.D.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Ha, E.-S.; Kim, M.-S. Current Status of Supersaturable Self-Emulsifying Drug Delivery Systems. Pharmaceutics 2020, 12, 365. [Google Scholar] [CrossRef] [Green Version]










| Formulation | Itraconazole | Poly(vinyl alcohol) | Copovidone | Crospovidone |
|---|---|---|---|---|
| PVA | 20% | 80% | - | - |
| PVA_K/VA | 56% | 24% | - | |
| PVA_K/CL | 76% | - | 4% |
| Filament Composition | Diameter ± SD (mm) | Itraconazole Content ± SD (%) | Tensile Strength ± SD (MPa) | Young’s Modulus ± SD (MPa) |
|---|---|---|---|---|
| PVA | 1.70 ± 0.02 | 19.67 ± 0.43 | 49.0 ± 10.3 | 2641.1 ± 144.4 |
| PVA_K/CL | 1.68 ± 0.07 | 19.60 ± 0.34 | 52.6 ± 19.8 | 2771.1 ± 347.2 |
| PVA_K/VA | 1.69 ± 0.05 | 19.20 ± 0.33 | 28.2 ± 7.1 | 2042.1 ± 256.3 |
| Sample | Tg (K) | TSm-N (K) | TN-I (K) |
|---|---|---|---|
| Neat ITR | 332 | 348 | 364 |
| PVA filament | 315 | 326 | 347 |
| PVA 3DP tablet | 306 | 330 | 344 |
| PVA_K/CL filament | 312 | 329 | 346 |
| PVA_K/CL 3DP tablet | 308 | 330 | 344 |
| PVA_K/VA filament | 317 | 328 (TSm-I) | |
| PVA_K/VA 3DP tablet | 315 | 330 (TSm-I) | |
| Polymers | Infill (%) | Mass (mg) | Width (mm) | Length (mm) | Height (mm) | Number of Layers | Real Layer Height (mm) |
|---|---|---|---|---|---|---|---|
| PVA | 35 | 252.82 ± 4.16 | 10.18 ± 0.03 | 20.15 ± 0.03 | 2.34 ± 0.03 | 16 | 0.142 |
| PVA_K/VA | 35 | 253.05 ± 3.67 | 10.08 ± 0.01 | 20.09 ± 0.01 | 2.89 ± 0.05 | 20 | 0.142 |
| PVA_K/CL | 35 | 250.12 ± 4.52 | 9.98 ± 0.02 | 19.85 ± 0.12 | 2.67 ± 0.03 | 17 | 0.154 |
| PVA_K/CL | 20 | 244.12 ± 5.77 | 9.96 ± 0.03 | 19.86 ± 0.09 | 3.65 ± 0.03 | 24 | 0.150 |
| PVA_K/CL | 60 | 239.73 ± 3.01 | 9.99 ± 0.05 | 20.05 ± 0.03 | 1.78 ± 0.02 | 11 | 0.158 |
| Description | Unit | T_20 | T_35 | T_60 |
|---|---|---|---|---|
| Object volume | mm3 | 236 | 220 | 202 |
| Percent object volume | % | 33 | 41 | 60 |
| Structure thickness | mm | 0.25 | 0.20 | 0.19 |
| Structure separation | mm | 1.11 | 0.62 | 0.25 |
| Volume of open pore space | mm3 | 485 | 312 | 134 |
| Open porosity | % | 67.2 | 58.5 | 39.9 |
| Description | Unit | T_35_1 | T_35_2 | T_35_3 |
|---|---|---|---|---|
| Object volume | mm3 | 220 | 224 | 213 |
| Percent object volume | % | 41 | 42 | 39 |
| Structure thickness | mm | 0.20 | 0.19 | 0.17 |
| Structure separation | mm | 0.62 | 0.62 | 0.62 |
| Volume of open pore space | mm3 | 312 | 307 | 327 |
| Open porosity | % | 58.5 | 57.7 | 60.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamróz, W.; Pyteraf, J.; Kurek, M.; Knapik-Kowalczuk, J.; Szafraniec-Szczęsny, J.; Jurkiewicz, K.; Leszczyński, B.; Wróbel, A.; Paluch, M.; Jachowicz, R. Multivariate Design of 3D Printed Immediate-Release Tablets with Liquid Crystal-Forming Drug—Itraconazole. Materials 2020, 13, 4961. https://doi.org/10.3390/ma13214961
Jamróz W, Pyteraf J, Kurek M, Knapik-Kowalczuk J, Szafraniec-Szczęsny J, Jurkiewicz K, Leszczyński B, Wróbel A, Paluch M, Jachowicz R. Multivariate Design of 3D Printed Immediate-Release Tablets with Liquid Crystal-Forming Drug—Itraconazole. Materials. 2020; 13(21):4961. https://doi.org/10.3390/ma13214961
Chicago/Turabian StyleJamróz, Witold, Jolanta Pyteraf, Mateusz Kurek, Justyna Knapik-Kowalczuk, Joanna Szafraniec-Szczęsny, Karolina Jurkiewicz, Bartosz Leszczyński, Andrzej Wróbel, Marian Paluch, and Renata Jachowicz. 2020. "Multivariate Design of 3D Printed Immediate-Release Tablets with Liquid Crystal-Forming Drug—Itraconazole" Materials 13, no. 21: 4961. https://doi.org/10.3390/ma13214961
APA StyleJamróz, W., Pyteraf, J., Kurek, M., Knapik-Kowalczuk, J., Szafraniec-Szczęsny, J., Jurkiewicz, K., Leszczyński, B., Wróbel, A., Paluch, M., & Jachowicz, R. (2020). Multivariate Design of 3D Printed Immediate-Release Tablets with Liquid Crystal-Forming Drug—Itraconazole. Materials, 13(21), 4961. https://doi.org/10.3390/ma13214961