Catalyst-Less and Transfer-Less Synthesis of Graphene on Si(100) Using Direct Microwave Plasma Enhanced Chemical Vapor Deposition and Protective Enclosures
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Raman Spectra of Directly Synthesized Graphene
3.2. Effect of Synthesis Conditions and Enclosure Design on the Graphene Structure
3.3. The Number of Graphene Layers and Defect Density
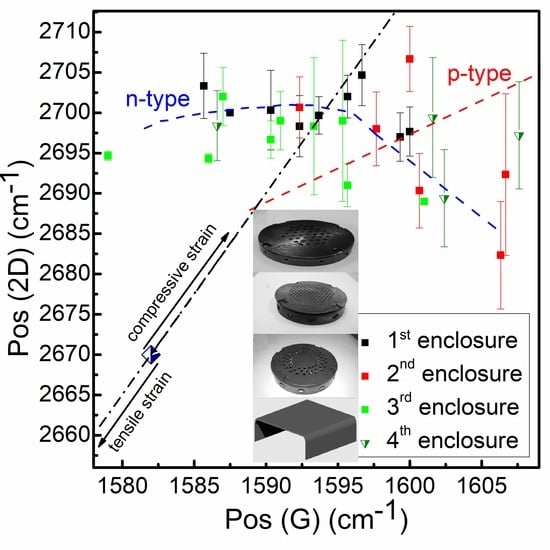
3.4. Dopant Density and Stress
3.5. AFM Study
4. Discussion
4.1. Effect of the Deposition Conditions. Comparison with Results Reported Elsewhere
4.2. Effect of the Deposition Conditions. Physical and Chemical Phenomena Involved
4.3. Effect of Protective Enclosure Design
4.4. Mechanisms Responsible to the N-Type Self Doping of Graphene and Induction of Compressive Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sattar, T. Current Review on Synthesis, Composites and Multifunctional Properties of Graphene. Top. Curr. Chem. 2019, 377, 10. [Google Scholar] [CrossRef]
- Banszerus, L.; Schmitz, M.; Engels, S.; Dauber, J.; Oellers, M.; Haupt, F.; Stampfer, C. Ultrahigh-mobility graphene devices from chemical vapor deposition on reusable copper. Sci. Adv. 2015, 1, e1500222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tielrooij, K.J.; Song, J.C.W.; Jensen, S.A.; Centeno, A.; Pesquera, A.; Zurutuza Elorza, A.; Bonn, M.; Levitov, L.S.; Koppens, F.H.L. Photoexcitation cascade and multiple hot-carrier generation in graphene. Nat. Phys. 2013, 9, 248–252. [Google Scholar] [CrossRef]
- Song, Y.; Fang, W.; Brenes, R.; Kong, J. Challenges and opportunities for graphene as transparent conductors in optoelectronics. Nano Today 2015, 10, 681–700. [Google Scholar] [CrossRef]
- Nayak, P.K. Pulsed-grown graphene for flexible transparent conductors. Nanoscale Adv. 2019, 1, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Yu, X.; Yang, D. A review on graphene-silicon Schottky junction interface. J. Alloys Compd. 2019, 806, 63–70. [Google Scholar] [CrossRef]
- Di Bartolomeo, A. Graphene Schottky diodes: An experimental review of the rectifying graphene/semiconductor heterojunction. Phys. Rep. 2016, 606, 1–58. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, M.; Mao, D.; Park, J.; Xu, G. Graphene field-effect transistors: The road to bioelectronics. J. Phys. D. Appl. Phys. 2018, 51, 493001. [Google Scholar] [CrossRef]
- Geng, H.; Yuan, D.; Yang, Z.; Tang, Z.; Zhang, X.; Yang, G.; Su, Y. Graphene van der Waals heterostructures for high-performance photodetectors. J. Mater. Chem. C 2019, 7, 11056–11067. [Google Scholar] [CrossRef]
- Rogalski, A. Graphene-based materials in the infrared and terahertz detector families: A tutorial. Adv. Opt. Photon. 2019, 11, 314–379. [Google Scholar] [CrossRef]
- Shin, D.; Choi, S.-H. Graphene-Based Semiconductor Heterostructures for Photodetectors. Micromachines 2018, 9, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, X.; Zhang, L.; Liu, B.; Gao, H.; Zhang, Y.; Yan, H.; Song, X. Graphene/Si Schottky solar cells: A review of recent advances and prospects. RSC. Adv. 2019, 9, 863–877. [Google Scholar] [CrossRef] [Green Version]
- Patil, K.; Rashidi, S.; Wang, H.; Wei, W. Recent Progress of Graphene-Based Photoelectrode Materials for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2019, 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.Z.; Rehman, A.-U. Recent progress in graphene incorporated solar cell devices. Sol. Energy 2018, 169, 634–647. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Haigh, S.J.; Gholinia, A.; Jalil, R.; Romani, S.; Britnell, L.; Elias, D.C.; Novoselov, K.S.; Ponomarenko, L.A.; Geim, A.K.; Gorbachev, R. Cross-sectional Imaging of Individual Layers and Buried Interfaces of Graphene-Based Heterostructures and Superlattices. Nat. Mater. 2012, 11, 764–767. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Khranovskyy, V.; Yakimova, R. Combining graphene with silicon carbide: Synthesis and properties—A review. Semicond. Sci. Technol. 2016, 31, 113004. [Google Scholar] [CrossRef] [Green Version]
- She, X.; Huang, A.Q.; Lucia, O.; Ozpineci, B. Review of Silicon Carbide Power Devices and Their Applications. IEEE Trans. Ind. Electron. 2017, 64, 8193–8205. [Google Scholar] [CrossRef]
- Khan, A.; Islam, S.M.; Ahmed, S.; Kumar, R.R.; Habib, M.R.; Huang, K.; Hu, M.; Yu, X.; Yang, D. Direct CVD Growth of Graphene on Technologically Important Dielectric and Semiconducting Substrates. Adv. Sci. 2018, 5, 1800050. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhong, G.; Wu, X.; D’Arsiè, L.; Robertson, J. Metal-catalyst-free growth of graphene on insulating substrates by ammonia-assisted microwave plasma-enhanced chemical vapor deposition. RSC Adv. 2017, 7, 33185–33193. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Deng, B.; Guo, X.; Chen, S.; Gao, J.; Li, T.; Dou, Z.; Ci, H.; Sun, J.; Chen, Z.; et al. Switching Vertical to Horizontal Graphene Growth Using Faraday Cage-Assisted PECVD Approach for High-Performance Transparent Heating Device. Adv. Mater. 2018, 30, 1704839. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Lin, Y.-H.; Hwang, J.-Y.; Chang, R.; Chattopadhyay, S.; Chen, C.-J.; Chen, P.; Chiang, H.-P.; Tsai, T.-R.; Chen, L.-C. Imaging layer number and stacking order through formulating Raman fingerprints obtained from hexagonal single crystals of few layer graphene. Nanotechnology 2012, 24, 015702. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Tian, J.; Chen, Y.P. Effect of oxygen plasma etching on graphene studied using Raman spectroscopy and electronic transport measurements. New J. Phys. 2011, 13, 025008. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A 2010, 368, 5355–5377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tan, P.H.; Liu, J.; Ferrari, A.C. Intercalation of Few-Layer Graphite Flakes with FeCl3: Raman Determination of Fermi Level, Layer by Layer Decoupling, and Stability. J. Am. Chem. Soc. 2011, 133, 5941–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szirmai, P.; Márkus, B.G.; Chacón-Torres, J.C.; Eckerlein, P.; Edelthalhammer, K.; Englert, J.M.; Mundloch, U.; Hirsch, A.; Hauke, F.; Náfrádi, B.; et al. Characterizing the maximum number of layers in chemically exfoliated graphene. Sci. Rep. 2019, 9, 19480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical separation of mechanical strain from charge doping in graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef] [Green Version]
- Sakavičius, A.; Astromskas, G.; Bukauskas, V.; Kamarauskas, M.; Lukša, A.; Nargelienė, V.; Niaura, G.; Ignatjev, I.; Treideris, M.; Šetkus, A. Long distance distortions in the graphene near the edge of planar metal contacts. Thin Solid Films 2020, 698, 137850. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, S. Thickness-dependent native strain in graphene membranes visualized by Raman spectroscopy. Carbon 2016, 100, 283–290. [Google Scholar] [CrossRef]
- Armano, A.; Buscarino, G.; Cannas, M.; Gelardi, F.M.; Giannazzo, F.; Schilirò, E.; Agnello, S. Monolayer graphene doping and strain dynamics induced by thermal treatments in controlled atmosphere. Carbon 2018, 127, 270–279. [Google Scholar] [CrossRef]
- Neumann, C.; Reichardt, S.; Venezuela, P.; Drögeler, M.; Banszerus, L.; Schmitz, M.; Watanabe, K.; Taniguchi, T.; Mauri, F.; Beschoten, B.; et al. Raman spectroscopy as probe of nanometre-scale strain variations in graphene. Nat. Commun. 2015, 6, 8429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, U.; Han, Y.; Lee, S.; Kim, J.S.; Lee, Y.H.; Kim, U.J.; Son, H. Time Evolution Studies on Strain and Doping of Graphene Grown on a Copper Substrate Using Raman Spectroscopy. ACS Nano 2020, 14, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Y.; Lo, C.-L.; Zhang, S.; Chen, Z.; Marconnet, A. Dynamically tunable thermal transport in polycrystalline graphene by strain engineering. Carbon 2020, 158, 63–68. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.G.; Lombardo, A.; Nair, R.R.; Bonetti, A.; Savini, G.; Jalil, R.; Bonini, N.; Basko, D.M.; Galiotis, C.; Marzari, N.; et al. Uniaxial strain in graphene by Raman spectroscopy: G peak splitting, Grüneisen parameters, and sample orientation. Phys. Rev. B 2009, 79, 205433. [Google Scholar] [CrossRef]
- Ni, Z.H.; Yu, T.; Lu, Y.H.; Wang, Y.Y.; Feng, Y.P.; Shen, Z.X. Uniaxial Strain on Graphene: Raman Spectroscopy Study and Band-Gap Opening. ACS Nano 2008, 2, 2301–2305. [Google Scholar] [CrossRef]
- Chugh, S.; Mehta, R.; Lu, N.; Dios, F.D.; Kim, M.J.; Chen, Z. Comparison of graphene growth on arbitrary non-catalytic substrates using low-temperature. Carbon 2015, 93, 393–399. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double Resonant Raman Scattering in Graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef]
- Zafar, Z.; Ni, Z.H.; Wu, X.; Shi, Z.X.; Nan, H.Y.; Bai, J.; Sun, L.T. Evolution of Raman spectra in nitrogen doped graphene. Carbon 2013, 61, 57. [Google Scholar] [CrossRef]
- Soin, N.; Roy, S.S.; O’Kane, C.; McLaughlin, J.A.D.; Lim, T.H.; Hetherington, C.J.D. Exploring the fundamental effects of deposition time on the microstructure of graphene nanoflakes by Raman scattering and X-ray diffraction. Cryst. Engl. Commun. 2011, 13, 312–318. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, R.; Munuera, C.; Martínez, J.I.; Azpeitia, J.; Gómez-Aleixandre, C.; García-Hernández, M. Low temperature metal free growth of graphene on insulating substrates by plasma assisted chemical vapor deposition. 2D Mater. 2017, 4, 015009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Man, B.; Jiang, S.; Yue, W.; Yang, C.; Liu, M.; Chen, C.; Zhang, C. Direct growth of graphene on quartz substrates for label-free detection of adenosine triphosphate. Nanotechnology 2014, 25, 165702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fox, L.; Włodek, M.; Islas, L.; Slastanova, A.; Robles, E.; Bikondo, O.; Harniman, R.; Fox, N.; Cattelan, M.; et al. Surface structure of few layer graphene. Carbon 2018, 136, 255–261. [Google Scholar] [CrossRef]
- Nemes-Incze, P.; Osváth, Z.; Kamarás, K.; Biró, L.P. Anomalies in thickness measurements of graphene and few layer graphite crystals by tapping mode atomic force microscopy. Carbon 2008, 46, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Yaxuan, Y.; Lingling, R.; Sitian, G.; Shi, L. Histogram method for reliable thickness measurements of graphene films using atomic force microscopy (AFM). J. Mater. Sci. Technol. 2017, 33, 815–820. [Google Scholar] [CrossRef]
- Shearer, C.J.; Slattery, A.D.; Stapleton, A.J.; Shapter, J.G.; Gibson, C.T. Accurate thickness measurement of graphene. Nanotechnology 2016, 27, 125704. [Google Scholar] [CrossRef]
- Kim, Y.S.; Joo, K.; Jerng, S.-K.; Lee, J.H.; Yoon, E.; Chun, S.-H. Direct growth of patterned graphene on SiO2 substrates without the use of catalysts or Lithography. Nanoscale 2014, 6, 10100. [Google Scholar] [CrossRef]
- Kalita, G.; Kayastha, M.S.; Uchida, H.; Wakita, K.; Umeno, M. Direct growth of nanographene films by surface wave plasma chemical vapor deposition and their application in photovoltaic devices. RSC Adv. 2012, 2, 3225–3230. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Bertran, E. Effect of temperature on graphene grown by chemical vapor deposition. J. Mater. Sci. 2017, 52, 8348–8356. [Google Scholar] [CrossRef]
- Scaparro, A.M.; Miseikis, V.; Coletti, C.; Notargiacomo, A.; Pea, M.; De Seta, M.; Di Gaspare, L. Investigating the CVD Synthesis of Graphene on Ge(100): Toward Layer-by-Layer Growth. ACS Appl. Mater. Interfaces 2016, 8, 33083–33090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Lee, J.H.; Kim, Y.D.; Jerng, S.-K.; Joo, K.; Kim, E.; Jung, J.; Yoon, E.; Park, Y.D.; Seo, S.; et al. Methane as an effective hydrogen source for single-layer graphene synthesis on Cu foil by plasma enhanced chemical vapor deposition. Nanoscale 2013, 5, 1221–1226. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Kavitha, K.; Lahiri, I. Transfer-Free Graphene Growth on Dielectric Substrates: A Review of the Growth Mechanism. Crit. Rev. Solid State Mater. Sci. 2019, 44, 157–209. [Google Scholar] [CrossRef]
- Park, H.J.; Meyer, J.; Roth, S.; Skákalová, V. Growth and properties of few-layer graphene prepared by chemical vapor deposition. Carbon 2010, 48, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Hug, D.; Zihlmann, S.; Rehmann, M.K.; Kalyoncu, Y.B.; Camenzind, T.N.; Marot, L.; Watanabe, K.; Taniguchi, T.; Zumbühl, D.M. Anisotropic etching of graphite and graphene in a remote hydrogen plasma. NPJ 2D Mater. Appl. 2017, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kiraly, B.; Jacobberger, R.M.; Mannix, A.J.; Campbell, G.P.; Bedzyk, M.J.; Arnold, M.S.; Hersam, M.C.; Guisinger, N.P. Electronic and Mechanical Properties of Graphene–Germanium Interfaces Grown by Chemical Vapor Deposition. Nano Lett. 2015, 15, 7414–7420. [Google Scholar] [CrossRef]
- Ryu, S.; Liu, L.; Berciaud, S.; Yu, Y.-J.; Liu, H.; Kim, P.; Flynn, G.W.; Brus, L.E. Atmospheric Oxygen Binding and Hole Doping in Deformed Graphene on a SiO2 Substrate. Nano Lett. 2010, 10, 4944–4951. [Google Scholar] [CrossRef] [Green Version]
- Casiraghi, C.; Pisana, S.; Novoselov, K.S.; Geim, A.K.; Ferrari, A.C. Raman fingerprint of charged impurities in graphene. Appl. Phys. Lett. 2007, 91, 233108. [Google Scholar] [CrossRef] [Green Version]
- Kolesov, E.A.; Tivanov, M.S.; Korolik, O.V.; Kapitanova, O.O.; Fu, X.; Cho, H.D.; Kang, T.W.; Panin, G.N. The effect of atmospheric doping on pressure-dependent Raman scattering in supported graphene. Beilstein J. Nanotechnol. 2018, 9, 704–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goniszewski, S.; Adabi, M.; Shaforost, O.; Hanham, S.M.; Hao, L.; Klein, N. Correlation of p-doping in CVD Graphene with Substrate Surface Charges. Sci. Rep. 2016, 6, 22858. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Puglisi, D.; Vasiliauskas, R.; Lloyd Spetz, A.; Yakimova, R. Thickness uniformity and electron doping in epitaxial graphene on SiC. Mater. Sci. Forum 2013, 740, 153–156. [Google Scholar] [CrossRef]
- Jee, H.-g.; Jin, K.-H.; Han, J.-H.; Hwang, H.-N.; Jhi, S.-H.; Kim, Y.D.; Hwang, C.-C. Controlling the self-doping of epitaxial graphene on SiC via Ar ion treatment. Phys. Rev. B 2011, 84, 075457. [Google Scholar] [CrossRef] [Green Version]
- Banszerus, L.; Janssen, H.; Otto, M.; Epping, A.; Taniguchi, T.; Watanabe, K.; Beschoten, B.; Neumaier, D.; Stampfer, C. Identifying suitable substrates for high-quality graphene-based heterostructures. 2D Mater. 2017, 4, 025030. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Kang, J.; Chang, K.J. Electronic structure of graphene and doping effect on SiO2. Phys. Rev. B. 2008, 78, 115404. [Google Scholar] [CrossRef] [Green Version]
- Armano, A.; Buscarino, G.; Cannas, M.; Gelardi, F.M.; Giannazzo, F.; Schiliro, E.; Lo Nigro, R.; Agnello, S. Graphene-SiO2 Interaction from Composites to Doping. Phys. Status Solidif. 2019, 216, 1800540. [Google Scholar] [CrossRef]
- Barbosa, A.N.; Ptak, F.; Mendoza, C.D.; Maia da Costa, M.E.H.; Freire, F.L., Jr. Direct synthesis of bilayer graphene on silicon dioxide substrates. Diam. Relat. Mater. 2019, 95, 71–76. [Google Scholar] [CrossRef]
- Chen, Z.; Qi, Y.; Chen, X.; Zhang, Y.; Liu, Z. Direct CVD Growth of Graphene on Traditional Glass: Methods and Mechanisms. Adv. Mater. 2018, 31, 1803639. [Google Scholar] [CrossRef]








| Sample No. | Enclosure No. | P, kW | H2, sccm | CH4, sccm | p, mBar | t, °C | t, min |
|---|---|---|---|---|---|---|---|
| 2E1 | 1 | 1.2 | 150 | 50 | 30 | 900 | 30 |
| 3E1 | 1 | 1.2 | 180 | 20 | 30 | 900 | 30 |
| 4E1 | 1 | 1.2 | 120 | 80 | 30 | 900 | 30 |
| 5E1 | 1 | 1.2 | 150 | 50 | 30 | 900 | 15 |
| 6E1 | 1 | 1.2 | 150 | 50 | 30 | 900 | 45 |
| 7E1 | 1 | 1.2 | 150 | 50 | 30 | 700 | 30 |
| 8E1 | 1 | 1.2 | 150 | 50 | 30 | 800 | 30 |
| 9E1 | 1 | 0.8 | 150 | 50 | 30 | 900 | 30 |
| 10E1 | 1 | 1.0 | 150 | 50 | 30 | 900 | 30 |
| 1E2 | 2 | 1.2 | 150 | 50 | 30 | 800 | 30 |
| 2E2 | 2 | 1.2 | 150 | 50 | 30 | 700 | 30 |
| 3E2 | 2 | 1.2 | 150 | 50 | 30 | 900 | 30 |
| 4E2 | 2 | 1.2 | 180 | 20 | 30 | 900 | 30 |
| 5E2 | 2 | 1.2 | 120 | 80 | 30 | 900 | 30 |
| 6E2 | 2 | 1.0 | 150 | 50 | 30 | 900 | 30 |
| 7E2 | 2 | 0.8 | 150 | 50 | 30 | 900 | 30 |
| 3E3 | 3 | 1.2 | 150 | 50 | 30 | 900 | 30 |
| 4E3 | 3 | 1.2 | 180 | 20 | 30 | 900 | 30 |
| 5E3 | 3 | 1.2 | 120 | 80 | 30 | 900 | 30 |
| 6E3 | 3 | 0.8 | 150 | 50 | 30 | 900 | 30 |
| 7E3 | 3 | 1.0 | 150 | 50 | 30 | 900 | 30 |
| 1E4 | 4 | 1.2 | 150 | 50 | 30 | 800 | 30 |
| 2E4 | 4 | 1.2 | 150 | 50 | 22 | 700 | 30 |
| 3E4 | 4 | 1.2 | 150 | 50 | 22 | 800 | 30 |
| 4E4 | 4 | 1.2 | 150 | 50 | 22 | 900 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudaitis, R.; Lazauskas, A.; Jankauskas, Š.; Meškinis, Š. Catalyst-Less and Transfer-Less Synthesis of Graphene on Si(100) Using Direct Microwave Plasma Enhanced Chemical Vapor Deposition and Protective Enclosures. Materials 2020, 13, 5630. https://doi.org/10.3390/ma13245630
Gudaitis R, Lazauskas A, Jankauskas Š, Meškinis Š. Catalyst-Less and Transfer-Less Synthesis of Graphene on Si(100) Using Direct Microwave Plasma Enhanced Chemical Vapor Deposition and Protective Enclosures. Materials. 2020; 13(24):5630. https://doi.org/10.3390/ma13245630
Chicago/Turabian StyleGudaitis, Rimantas, Algirdas Lazauskas, Šarūnas Jankauskas, and Šarūnas Meškinis. 2020. "Catalyst-Less and Transfer-Less Synthesis of Graphene on Si(100) Using Direct Microwave Plasma Enhanced Chemical Vapor Deposition and Protective Enclosures" Materials 13, no. 24: 5630. https://doi.org/10.3390/ma13245630
APA StyleGudaitis, R., Lazauskas, A., Jankauskas, Š., & Meškinis, Š. (2020). Catalyst-Less and Transfer-Less Synthesis of Graphene on Si(100) Using Direct Microwave Plasma Enhanced Chemical Vapor Deposition and Protective Enclosures. Materials, 13(24), 5630. https://doi.org/10.3390/ma13245630