The Effect of the Gaseous Environment on the Electrical Conductivity of Multi-Walled Carbon Nanotube Films over a Wide Temperature Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CNT Films
2.2. Assessing the Electrothermal Properties
2.3. Electrical Measurements under Non-Ambient Conditions
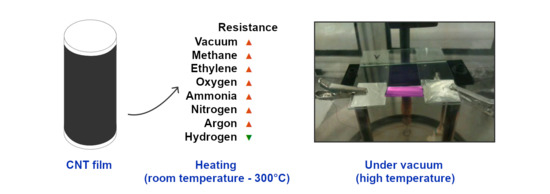
2.3.1. Vacuum Experiments
2.3.2. Artificial Atmosphere Experiments
2.4. Characterization
3. Results
3.1. Experiments in Vacuum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Brady, G.J.; Way, A.J.; Safron, N.S.; Evensen, H.T.; Gopalan, P.; Arnold, M.S. Quasi-ballistic carbon nanotube array transistors with current density exceeding Si and GaAs. Sci. Adv. 2016, 2, e1601240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Wang, C. Carbon Nanotube Flexible and Stretchable Electronics. Nanoscale Res. Lett. 2015, 10, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: A review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef] [Green Version]
- Arash, B.; Wang, Q.; Varadan, V.K. Mechanical properties of carbon nanotube/polymer composites. Sci. Rep. 2014, 4, 6479. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.D.; Wang, X. Nonlinear pull-in instability of carbon nanotubes reinforced nano-actuator with thermally corrected Casimir force and surface effect. Int. J. Mech. Sci. 2016, 107, 34–42. [Google Scholar] [CrossRef]
- Yu, W.-J.; Liu, C.; Hou, P.-X.; Zhang, L.; Shan, X.-Y.; Li, F.; Cheng, H.-M. Lithiation of Silicon Nanoparticles Confined in Carbon Nanotubes. ACS Nano 2015, 9, 5063–5071. [Google Scholar] [CrossRef]
- Arash, B.; Wang, Q. Detection of gas atoms with carbon nanotubes. Sci. Rep-Uk 2013, 3, 1782. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.-T.; Lin, Z.; Zheng, S.-Y.; Terrones, M. A carbon nanotube integrated microfluidic device for blood plasma extraction. Sci. Rep. 2018, 8, 13623. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Ma, Z.; Wei, N.; Liu, H.; Wang, S.; Peng, L.-M. Solid state carbon nanotube device for controllable trion electroluminescence emission. Nanoscale 2016, 8, 6761–6769. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, D.; Yang, X.; Yuan, L.; Li, H.; Wang, J.; Chen, M.; Deng, G.; Liang, W.; Li, Q.; et al. Stressed carbon nanotube devices for high tunability, high quality factor, single mode GHz resonators. Nano Res. 2018, 11, 5812–5822. [Google Scholar] [CrossRef]
- Ericson, L.M.; Fan, H.; Peng, H.Q.; Davis, V.A.; Zhou, W.; Sulpizio, J.; Wang, Y.H.; Booker, R.; Vavro, J.; Guthy, C.; et al. Macroscopic, neat, single-walled carbon nanotube fibers. Science 2004, 305, 1447–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.L.; Kinloch, I.A.; Windle, A.H. Direct spinning of carbon nanotube fibers from chemical vapor deposition synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Atkinson, K.R.; Baughman, R.H. Multifunctional carbon nanotube yarns by downsizing an ancient technology. Science 2004, 306, 1358–1361. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. Rapid electrothermal response of high-temperature carbon nanotube film heaters. Carbon 2013, 59, 457–463. [Google Scholar] [CrossRef]
- Aliev, A.E.; Gartstein, Y.N.; Baughman, R.H. Mirage effect from thermally modulated transparent carbon nanotube sheets. Nanotechnology 2011, 22, 435704. [Google Scholar] [CrossRef] [Green Version]
- Butt, H.; Montelongo, Y.; Butler, T.; Rajesekharan, R.; Dai, Q.; Shiva-Reddy, S.G.; Wilkinson, T.D.; Amaratunga, G.A.J. Carbon Nanotube Based High Resolution Holograms. Adv. Opt. Mater. 2012, 24, 331–336. [Google Scholar] [CrossRef]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Worsley, M.A.; Stadermann, M.; Wang, Y.M.M.; Satcher, J.H.; Baumann, T.F. High surface area carbon aerogels as porous substrates for direct growth of carbon nanotubes. Chem. Commun. 2010, 46, 9253–9255. [Google Scholar] [CrossRef]
- Xiao, M.; Liang, S.; Han, J.; Zhong, D.; Liu, J.; Zhang, Z.; Peng, L. Batch Fabrication of Ultrasensitive Carbon Nanotube Hydrogen Sensors with Sub-ppm Detection Limit. ACS Sens. 2018, 3, 749–756. [Google Scholar] [CrossRef]
- Rajavel, K.; Lalitha, M.; Radhakrishnan, J.K.; Senthilkumar, L.; Rajendra Kumar, R.T. Multiwalled Carbon Nanotube Oxygen Sensor: Enhanced Oxygen Sensitivity at Room Temperature and Mechanism of Sensing. ACS Appl. Mater. Interfaces 2015, 7, 23857–23865. [Google Scholar] [CrossRef] [PubMed]
- Chimowa, G.; Tshabalala, Z.P.; Akande, A.A.; Bepete, G.; Mwakikunga, B.; Ray, S.S.; Benecha, E.M. Improving methane gas sensing properties of multi-walled carbon nanotubes by vanadium oxide filling. Sens. Actuators B Chem. 2017, 247, 11–18. [Google Scholar] [CrossRef]
- Agarwal, P.B.; Alam, B.; Sharma, D.S.; Sharma, S.; Mandal, S.; Agarwal, A. Flexible NO2 gas sensor based on single-walled carbon nanotubes on polytetrafluoroethylene substrates. Flex. Print. Electron. 2018, 3, 035001. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, H.; Gui, Y.; Tang, J. Mechanism and Application of Carbon Nanotube Sensors in SF6 Decomposed Production Detection: A Review. Nanoscale Res. Lett. 2017, 12, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Septiani, N.L.W.; Yuliarto, B. The development of gas sensor based on carbon nanotubes. J. Electrochem. Soc. 2016, 163, B97–B106. [Google Scholar] [CrossRef]
- Young, S.; Lin, Z. Ammonia gas sensors with Au-decorated carbon nanotubes. Microsyst. Technol. 2018, 24, 4207–4210. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. Carbon nanotube fibers and films: Synthesis, applications and perspectives of the direct-spinning method. Nanoscale 2016, 8, 19475–19490. [Google Scholar] [CrossRef]
- Lekawa-Raus, A.; Kurzepa, L.; Kozlowski, G.; Hopkins, S.C.; Wozniak, M.; Lukawski, D.; Glowacki, B.A.; Koziol, K.K. Influence of atmospheric water vapour on electrical performance of carbon nanotube fibres. Carbon 2015, 87, 18–28. [Google Scholar] [CrossRef]
- Jung, S.M.; Jung, H.Y.; Dresselhaus, M.S.; Jung, Y.J.; Kong, J. A facile route for 3D aerogels from nanostructured 1D and 2D materials. Sci. Rep. 2012, 2, 849. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas Solid Systems with Special Reference to the Determination of Surface-Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y. Effects of activation conditions on BET specific surface area of activated carbon nanotubes. Microporous Mesoporous Mater. 2004, 76, 215–219. [Google Scholar] [CrossRef]
- Moonoosawmy, K.R.; Kruse, P. Cause and Consequence of Carbon Nanotube Doping in Water and Aqueous Media. J. Am. Chem. Soc. 2010, 132, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Zahab, A.; Spina, L.; Poncharal, P.; Marliere, C. Water-vapor effect on the electrical conductivity of a single-walled carbon nanotube mat. Phys. Rev. B 2000, 62, 10000–10003. [Google Scholar] [CrossRef]
- Roch, A.; Greifzu, M.; Talens, E.R.; Stepien, L.; Roch, T.; Hege, J.; Van Nong, N.; Schmiel, T.; Dani, I.; Leyens, C.; et al. Ambient effects on the electrical conductivity of carbon nanotubes. Carbon 2015, 95, 347–353. [Google Scholar] [CrossRef]
- Chaban, V.V.; Prezhdo, V.V.; Prezhdo, O.V. Confinement by Carbon Nanotubes Drastically Alters the Boiling and Critical Behavior of Water Droplets. ACS Nano 2012, 6, 2766–2773. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.Q.; Zhu, H.W.; Wu, D.H.; Wei, B.Q. Carbon nanotube filaments in household light bulbs. Appl. Phys. Lett. 2004, 84, 4869–4871. [Google Scholar] [CrossRef]
- Janas, D.; Sundaram, R.; Koziol, K.K.K. Surface modification of directly spun carbon nanotube films. Mater Lett. 2012, 79, 32–34. [Google Scholar] [CrossRef]
- Stando, G.; Łukawski, D.; Lisiecki, F.; Janas, D. Intrinsic hydrophilic character of carbon nanotube networks. Appl. Surf. Sci. 2019, 463, 227–233. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. Improved Performance of Ultra-Fast Carbon Nanotube Film Heaters. J. Autom. Control Eng. Vol 2014, 2, 150–153. [Google Scholar] [CrossRef]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown. Science 2001, 292, 706. [Google Scholar] [CrossRef]
- Collins, P.G.; Hersam, M.; Arnold, M.; Martel, R.; Avouris, P. Current Saturation and Electrical Breakdown in Multiwalled Carbon Nanotubes. Phys. Rev. Lett. 2001, 86, 3128–3131. [Google Scholar] [CrossRef] [PubMed]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.M.; Dresselhaus, M.S. Hydrogen storage in single-walled carbon nanotubes at room temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.-M.; Yang, Q.-H.; Liu, C. Hydrogen storage in carbon nanotubes. Carbon 2001, 39, 1447–1454. [Google Scholar] [CrossRef]
- Sun, Y.G.; Wang, H.H. High-performance, flexible hydrogen sensors that use carbon nanotubes decorated with palladium nanoparticles. Adv. Mater. 2007, 19, 2818–2823. [Google Scholar] [CrossRef]
- Khalap, V.R.; Sheps, T.; Kane, A.A.; Collins, P.G. Hydrogen Sensing and Sensitivity of Palladium-Decorated Single-Walled Carbon Nanotubes with Defects. Nano Lett. 2010, 10, 896–901. [Google Scholar] [CrossRef] [Green Version]
- Ganzhorn, M.; Vijayaraghavan, A.; Dehm, S.; Hennrich, F.; Green, A.A.; Fichtner, M.; Voigt, A.; Rapp, M.; von Lohneysen, H.; Hersam, M.C.; et al. Hydrogen Sensing with Diameter- and Chirality-Sorted Carbon Nanotubes. ACS Nano 2011, 5, 1670–1676. [Google Scholar] [CrossRef]
- Ventura, D.N.; Li, S.; Baker, C.A.; Breshike, C.J.; Spann, A.L.; Strouse, G.F.; Kroto, H.W.; Acquah, S.F.A. A flexible cross-linked multi-walled carbon nanotube paper for sensing hydrogen. Carbon 2012, 50, 2672–2674. [Google Scholar] [CrossRef]
- Rumiche, F.; Wang, H.H.; Indacochea, J.E. Development of a fast-response/high-sensitivity double wall carbon nanotube nanostructured hydrogen sensor. Sens. Actuators B Chem. 2012, 163, 97–106. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Cho, K. Ab initio study of hydrogen interaction with pure and nitrogen-doped carbon nanotubes. Phys. Rev. B 2007, 75, 075420. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Luzan, S.; Anoshkin, I.V.; Nasibulin, A.G.; Jiang, H.; Kauppinen, E.I.; Mikoushkin, V.M.; Shnitov, V.V.; Marchenko, D.E.; Noreus, D. Hydrogenation, Purification, and Unzipping of Carbon Nanotubes by Reaction with Molecular Hydrogen: Road to Graphane Nanoribbons. ACS Nano 2011, 5, 5132–5140. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.W.; Bando, Y.; Li, M.S.; Liu, Y.X.; Qi, Y.X. Unique single-crystalline beta carbon nitride nanorods. Adv. Mater. 2003, 15, 1840–1844. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Luo, G.H.; Wei, F. 99.9% purity multi-walled carbon nanotubes by vacuum high-temperature annealing. Carbon 2003, 41, 2585–2590. [Google Scholar] [CrossRef]
- Jung, S.I.; Jo, S.H.; Moon, H.S.; Kim, J.M.; Zang, D.S.; Lee, C.J. Improved crystallinity of double-walled carbon nanotubes after a high-temperature thermal annealing and their enhanced field emission properties. J. Phys. Chem. C 2007, 111, 4175–4179. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janas, D.; Koziol, K.K. The Effect of the Gaseous Environment on the Electrical Conductivity of Multi-Walled Carbon Nanotube Films over a Wide Temperature Range. Materials 2020, 13, 510. https://doi.org/10.3390/ma13030510
Janas D, Koziol KK. The Effect of the Gaseous Environment on the Electrical Conductivity of Multi-Walled Carbon Nanotube Films over a Wide Temperature Range. Materials. 2020; 13(3):510. https://doi.org/10.3390/ma13030510
Chicago/Turabian StyleJanas, Dawid, and Krzysztof K. Koziol. 2020. "The Effect of the Gaseous Environment on the Electrical Conductivity of Multi-Walled Carbon Nanotube Films over a Wide Temperature Range" Materials 13, no. 3: 510. https://doi.org/10.3390/ma13030510
APA StyleJanas, D., & Koziol, K. K. (2020). The Effect of the Gaseous Environment on the Electrical Conductivity of Multi-Walled Carbon Nanotube Films over a Wide Temperature Range. Materials, 13(3), 510. https://doi.org/10.3390/ma13030510