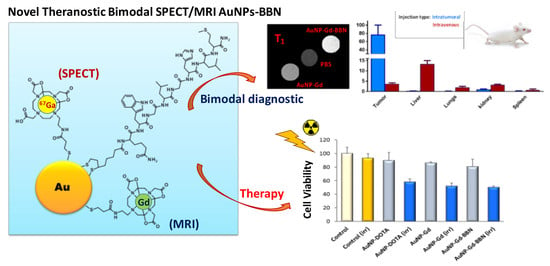
Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Synthesis of AuNP-TDOTA
2.3. Synthesis of AuNP-Gd
2.4. Synthesis of AuNP-Gd-BBN
2.5. Determination of the Amount of Conjugated TA-BBN
2.6. Determination of Gadolinium and Gold Content by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
2.7. Dynamic Light Scattering (DLS) and Zeta Potential Determination
2.8. Transmission Electron Microscopy
2.9. Radiolabeling with 67Ga3+
2.10. NMRD Profile
2.11. MRI Phantoms
2.12. Cytotoxicity Studies
2.13. Cellular Internalization Studies
2.14. Irradiation Setup
2.15. Biodistribution Studies
3. Results
3.1. Synthesis, Characterisation and Radiolabelling of the AuNPs
3.2. Relaxometric Studies
3.3. Cellular Internalization Studies
3.4. Radiosensitization Studies
3.5. Biodistribution Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ehlerding, E.B.; Sun, L.; Lan, X.; Zeng, D.; Cai, W. Dual-Targeted Molecular Imaging of Cancer. J. Nucl. Med. 2018, 59, 390–395. [Google Scholar] [CrossRef]
- Farolfi, A.; Lima, G.M.; Oyen, W.; Fanti, S. Molecular Imaging and Theranostics—A Multidisciplinary Approach. Semin. Nucl. Med. 2019, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Degrauwe, N.; Hocquele, A.; Digklia, A.; Schaefer, N.; Denys, A.; Duran, R. Theranostics in Interventional Oncology: Versatile Carriers for Diagnosis and Targeted Image-Guided Minimally Invasive Procedures. Front. Pharmacol. 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, G. Applications of Gold Nanoparticles in Cancer Imaging and Treatment. In Noble and Precious Metals—Properties, Nanoscale Effects and Applications; Seehra, M.S., Bristow, A.D., Eds.; Intechopen: London, UK, 2018; Chapter 13; pp. 291–309. [Google Scholar] [CrossRef]
- Mukherjee, A.; Paul, M.; Mukherjee, S. Recent Progress in the Theranostics Application of Nanomedicine in Lung Cancer. Cancers 2019, 11, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, M.; Sousa, J.J.; Pais, A.; Vitorino, C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics 2018, 10, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.R.; Gambhir, S. Nanomaterials for in vivo Imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Paliwal, S. A Review on Targeted Drug Delivery: Its Entire Focus on Advanced Therapeutics and Diagnostics. SJAMS 2014, 2, 328–331. [Google Scholar]
- Guo, J.; Rahme, K.; Hel, Y.; Li, L.-L.; Holmes, J.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131–6152. [Google Scholar] [CrossRef] [Green Version]
- Rajeeva, B.B.; Menz, R.; Zheng, Y. Towards rational design of multifunctional theranostic nanoparticles: What barriers do we need to overcome. Nanomedicine 2014, 9, 1767–1770. [Google Scholar] [CrossRef] [Green Version]
- Tóth, E.; Helm, L.; Merbach, A. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; John Wiley & Sons, Ltd.: Chichester, UK, 2013; Chapter 2; pp. 25–81. [Google Scholar]
- Wallyn, J.; Anton, N.; Akram, S.; Vandamme, T.F. Biomedical Imaging: Principles, Technologies, Clinical Aspects, Contraste agents, limitations and future trends in Nanomedicines. Pharm. Res. 2019, 36, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Peng, H.; Li, P.; Xu, Z.; Whittaker, A.K. The evolution of gadolinium based contrast agents: From single-modality to multi-modality. Nanoscale 2016, 8, 10491–10510. [Google Scholar] [CrossRef] [PubMed]
- Sahraei, Z.; Mirabzadeh, M.; Fadaei Fouladi, D.; Eslami, N.; Eshraghi, A. Magnetic Resonance Imaging Contrast Agents: A Review of Literature. J. Pharm. Care 2014, 2, 177–182. [Google Scholar]
- Zhou, Z.; Lu, Z.R. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip. Rev. 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef]
- Marangonia, V.S.; Neumann, O.; Hendersona, L.; Kaffese, C.C.; Zhangd, H.; Zhang, R.; Bishnoia, S.; Ayala-Orozcoa, C.; Zucolotto, V.; Banksone, J.A.; et al. Enhancing T1 magnetic resonance imaging contrast with internalized gadolinium(III) in a multilayer nanoparticle. Proc. Natl. Acad. Sci. USA 2017, 114, 6960–6965. [Google Scholar] [CrossRef] [Green Version]
- Tweedle, M.F.; Kumar, K. Magnetic Resonance Imaging (MRI) Contrast Agents. In Metallopharmaceuticals II, Topics in Biological Inorganic Chemistry; Clarke, M.J., Sadler, P.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 2, pp. 1–43. [Google Scholar] [CrossRef]
- Derlin, T.; Grünwald, V.; Steinbach, J.; Wester, H.J.; Ross, T.L. Molecular Imaging in Oncology Using Positron Emission Tomography. Dtsch. Arztebl. Int. 2018, 115, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Gallamini, A.; Zwarthoed, C.; Borra, A. Tomography (PET) in Oncology. Cancers 2014, 6, 1821–1889. [Google Scholar] [CrossRef] [Green Version]
- Bettinardi, V.; Castiglioni, I.; De Bernardi, E.; Gilardi, M.C. PET quantification: Strategies for partial volume correction. Clin. Trans. Imaging 2014, 2, 199–218. [Google Scholar] [CrossRef]
- Mahan, M.M.; Doiron, A.L. Gold Nanoparticles as X-Ray, CT, and Multimodal Imaging Contrast Agents: Formulation, Targeting, and Methodology. J. Nanomater. 2018, 2018, 5837276. [Google Scholar] [CrossRef]
- Şologan, M.; Padelli, F.; Giachetti, I.; Aquino, D.; Boccalon, M.; Adami, G.; Pengo, P.; Pasquato, L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials 2019, 9, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrana, A.A.; Aghanejadb, A.; Farajollahia, A.; Bararb, J.; Omidib, Y. Gold nanoparticles for radiosensitizing and imaging of cancer cells. Radiat. Phys. Chem. 2018, 152, 137–144. [Google Scholar] [CrossRef]
- Chabloz, N.G.; Wenzel, M.N.; Perry, H.L.; Yoon, C.; Molisso, S.; Stasiuk, G.; Elson, D.S.; Cass, A.E.G.; Wilton-Ely, J.D.E.T. Polyfunctionalised Nanoparticles Bearing Robust Gadolinium Surface Units for High Relaxivity Performance in MRI. Chem. Eur. J. 2019, 25, 10895–10906. [Google Scholar] [CrossRef] [PubMed]
- Miladi, I.; Alric, C.; Dufort, S.; Mowat, P.; Dutour, A.; Mandon, C.; Laurent, G.; Bräuer-Krisch, E.; Herath, N.; Coll, J.-L.; et al. The in Vivo Radiosensitizing Effect of Gold Nanoparticles Based MRI Contrast Agents. Small 2014, 10, 1116–1124. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef]
- Rostami, A.; Sazgarnia, A. Gold nanoparticles as cancer theranostic agents. Nanomed. J. 2019, 6, 147–160. [Google Scholar] [CrossRef]
- Sah, B.; Antosh, M.P. Effect of size on gold nanoparticles in radiation therapy: Uptake and survival effects. J. Nano Med. 2019, 2, 1013. [Google Scholar]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current Trends in Cancer Nanotheranostics: Metallic Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Beeler, E.; Gabani, P.; Singh, O.V. Implementation of nanoparticles in therapeutic radiation oncology. J. Nanopart. Res. 2017, 19, 179. [Google Scholar] [CrossRef]
- Yang, C.; Bromma, K.; Sung, W.; Schuemann, J.; Chithrani, D. Determining the Radiation Enhancement Effects of Gold Nanoparticles in Cells in a Combined Treatment with Cisplatin and Radiation at Therapeutic Megavoltage Energies. Cancers 2018, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W.; Li, Q. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef] [PubMed]
- Fauzia, R.P.; Denkova, A.G.; Djanashvili, K. Potential of MRI in Radiotherapy Mediated by Small Conjugates and Nanosystems. Inorganics 2019, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, R.J.; Rammohan, N.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Meade, T.J. Gd(III)-Dithiolane Gold Nanoparticles for T1-Weighted Magnetic Resonance Imaging of the Pancreas. Nano Lett. 2016, 16, 3202–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arami, H.; Patel, C.; Madsen, S.J.; Dickinson, P.J.; Davis, R.M.; Zeng, Y.; Sturges, B.K.; Woolard, K.D.; Habte, F.G.; Akin, D.; et al. Nanomedicine for Spontaneous Brain Tumors: A Companion Clinical Trial. ACS Nano 2019, 13, 2858–2869. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [Green Version]
- Raghvendra, R.; Kanthadeivi, A.; Sathesh, K.A.; Aarrthy, M.A. Diagnostics and therapeutic application of gold nanoparticles. Medicine 2014, 2, 4. [Google Scholar]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Aminabad, N.S.; Farshbaf, M.; Akbarzadeh, A. Recent Advances of Gold Nanoparticles in Biomedical Applications: State of the Art. Cell Biochem. Biophys. 2019, 77, 123–137. [Google Scholar] [CrossRef]
- Wyatt, L.C.; Lewis, J.; Oleg, A.; Reshetnyak, Y.K.; Engelman, D.M. Applications of pHLIP Technology for Cancer Imaging and Therapy. Trends Biotechnol. 2017, 35, 653–664. [Google Scholar] [CrossRef]
- Depciuch, J.; Stec, M.; Maximenko, A.; Pawlyta, M.; Baran, J.; Parlinska-Wojtan, M. Control of Arms of Au Stars Size and its Dependent Cytotoxicity and Photosensitizer Effects in in Photothermal Anticancer Therapy. Int. J. Mol. Sci. 2019, 20, 5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depciuch, J.; Stec, M.; Klebowski, B.; Baran, J.; Parlinska-Wojtan, M. Platinum–gold nanoraspberries as effective photosensitizer in anticancer photothermal therapy. J. Nanobiotechnol. 2019, 17, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depciuch, J.; Stec, M.; Maximenko, A.; Klebowski, B.; Baran, J.; Parlinska-Wojtan, M. Gold nanodahlias: Potential nanophotosensitizer in photothermal anticancer therapy. J. Mater. Sci. 2020, 55, 2530–2543. [Google Scholar] [CrossRef]
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. A 2019, 107, 251–285. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Mancilla, N.; Ferro-Flores, G.; Santos-Cuevas, C.; Ocampo-García, B.; Luna-Gutiérrez, M.; Azorín-Vega, E.; Isaac-Olivé, K.; Camacho-López, M.; Torres-García, E. Multifunctional targeted therapy system based on 99mTc/177Lu-labeled gold nanoparticles-Tat(49–57)-Lys3-bombesin internalized in nuclei of prostate cancer cells. J. Labelled Comp. Radiopharm. 2013, 56, 663–671. [Google Scholar] [CrossRef]
- Valetti, S.; Maione, F.; Mura, S.; Stella, B.; Desmaële, D.; Noiray, M.; Vergnaud, J.; Vauthier, C.; Cattel, L.; Giraudo, E.; et al. Peptide-functionalized nanoparticles for selective targeting of pancreatic tumor. J. Control Release 2014, 192, 29–39. [Google Scholar] [CrossRef]
- Hosta-Rigau, L.; Olmedo, I.; Arbiol, J.; Cruz, L.J.; Kogan, M.J.; Albericio, F. Multifunctionalized gold nanoparticles with peptides targeted to gastrin-releasing peptide receptor of a tumor cell line. Bioconjugate Chem. 2010, 21, 1070–1078. [Google Scholar] [CrossRef]
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.; Gano, L.; Campello, M.P.C.; Marques, R.; Prudencio, I.; Zambre, A.; Upendran, A.; Paulo, A.; Kannan, R. In vitro/in vivo “peeling” of multilayered aminocarboxylate gold nanoparticles evidenced by a kinetically stable Tc-99m-label. Dalton Trans. 2017, 46, 14572–14583. [Google Scholar] [CrossRef]
- Silva, F.; Zambre, A.; Campello, M.P.C.; Gano, L.; Santos, I.; Ferraria, A.M.; Ferreira, M.J.; Singh, A.; Upendran, A.; Paulo, A.; et al. Interrogating the Role of Receptor-Mediated Mechanisms: Biological Fate of Peptide-Functionalized Radiolabeled Gold Nanoparticles in Tumor Mice. Bioconjugate Chem. 2016, 27, 1153–1164. [Google Scholar] [CrossRef]
- Tsopelas, C. A study of radiogallium aqueous chemistry: In vitro and in vivo characterisation of 67Ga-hydrolysed-stannous fluoride particles. J. Label Compd. Radiopharm 2016, 59, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.M.S.; Faneca, H.; Almeida, J.A.S.; Pais, A.; Marques, E.F.; Lima, M.C.P.; Jurado, A.S. Gemini surfactant dimethylene-1,2-bis(tetradecyldimethylammonium bromide)-based gene vectors: A biophysicalapproach to transfection efficiency. Biochim. Biophys. Acta Biomembr. 2011, 1808, 341–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barge, A.; Cravotto, G.; Gianolio, E.; Fedeli, F. How to determine free Gd and free ligand in solution of Gd chelates. A technical note. Contrast Med. Mol. Imaging 2006, 1, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Rammohan, N.; Holbrook, R.J.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Carney, C.E.; Reichova, V.; Meade, T.J. Gd(III)-Gold Nanoconjugates Provide Remarkable Cell Labeling for High Field Magnetic Resonance Imaging. Bioconjugate Chem. 2017, 28, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Geraldes, C.F.G.C.; Laurent, S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Gonçalves, J.; Mousavi, B.; Prata, M.I.M.; Rodrigues, S.P.J.; Calle, D.; López-Larrubia, P.; Cerdan, S.; Rodrigues, T.B.; Ferreira, P.M.; et al. Gold nanoparticles functionalised with fast water exchanging Gd3+ chelates: Linker effects on the relaxivity. Dalton Trans. 2015, 44, 4016–4031. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, F.J.; Rotz, M.W.; Ghuman, H.; MacRenaris, K.W.; Meade, T.J.; Modo, M. DNA-gadolinium-gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells. Biomaterials 2016, 77, 291–306. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Peters, J.A.; Mayer, F.; Helm, L.; Djanashvili, K. Prototropic Exchange Governs T1 and T2 Relaxivities of a Potential MRI Contrast Agent Nanozeolite Gd−LTL with a High pH Responsiveness. J. Phys. Chem. C 2015, 119, 5080–5089. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- Cui, L.; Her, S.; Borst, G.R.; Bristow, R.G.; Jaffray, D.A.; Allen, C. Radiosensitization by gold nanoparticles: Will they ever make it to the clinic? Radiother. Oncol. 2017, 124, 344–356. [Google Scholar] [CrossRef]
- Dufort, S.; Le Duc, G.; Salomé, M.; Bentivegna, V.; Sancey, L.; Bräuer-Krisch, E.; Requardt, H.; Lux, F.; Coll1, J.-L.; Perriat, P.; et al. The High Radiosensitizing Efficiency of a Trace of Gadolinium-Based Nanoparticles in Tumors. Sci. Rep. 2016, 6, 29678. [Google Scholar] [CrossRef] [PubMed]
- Konoeda, H.; Takizawa, H.; Gower, A.; Zhao, M.; Adeyi, O.A.; Liu, M. Pharmacokinetics, tissue distribution and safety of gold nanoparticle/PKC Delta inhibitor peptide hybrid in rats. Nanotoxicology 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- David, M.E.; Grumezescu, A.M. Tailored Gold Nanoparticles for Cancer Imaging and Therapy. Mater. Int. 2019, 1, 13–24. [Google Scholar] [CrossRef]
- Melancon, M.P.; Lu, W.; Yang, Z.; Zhang, R.; Cheng, Z.; Elliot, A.M.; Stafford, J.; Olson, T.; Zhang, J.Z.; Li, C. In vitro and in vivo targeting of hollow gold nanoshells directed at epidermal growth factor receptors for photothermal ablation therapy. Mol. Cancer Ther. 2008, 7, 1730–1739. [Google Scholar] [CrossRef] [Green Version]
- Ng, Q.K.T.; Olariu, C.I.; Yaffee, M.; Taelman, V.F.; Marincek, N.; Krause, T.; Meier, L.; Walter, M.A. Indium-111 labeled gold nanoparticles for in-vivo molecular targeting. Biomaterials 2014, 35, 7050–7057. [Google Scholar] [CrossRef]






| Ratio AuNP:Gd | [Au] (mM) | [Gd] (mM) |
|---|---|---|
| 1:0.05 | 0.452 | 0.025 |
| 1:0.10 | 0.442 | 0.042 |
| 1:0.25 | 0.467 | 0.245 |
| 1:0.50 | 0.068 | 0.229 |
| 1:5.00 | 0.045 | 0.183 |
| Compound | Hydrodynamic Size (PDI) (nm) | Zeta Potential (mV) | Ratio (M) Au/Gd | Ratio (M) Au/BBN |
|---|---|---|---|---|
| AuNP-DOTA | 30.9 (0.6) | −43.7 ± 13.1 | --- | --- |
| AuNP-Gda | 47.3 (0.4) | −35.1 ± 14.1 | 1.9 | --- |
| AuNP-Gd-BBNa | 78.8 (0.9) | −10.9 ± 5.1 | 3.2 | 4.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.; Paulo, A.; Pallier, A.; Même, S.; Tóth, É.; Gano, L.; Marques, F.; Geraldes, C.F.G.C.; Castro, M.M.C.A.; Cardoso, A.M.; et al. Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials 2020, 13, 513. https://doi.org/10.3390/ma13030513
Silva F, Paulo A, Pallier A, Même S, Tóth É, Gano L, Marques F, Geraldes CFGC, Castro MMCA, Cardoso AM, et al. Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials. 2020; 13(3):513. https://doi.org/10.3390/ma13030513
Chicago/Turabian StyleSilva, Francisco, António Paulo, Agnès Pallier, Sandra Même, Éva Tóth, Lurdes Gano, Fernanda Marques, Carlos F.G.C. Geraldes, M. Margarida C.A. Castro, Ana M. Cardoso, and et al. 2020. "Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics" Materials 13, no. 3: 513. https://doi.org/10.3390/ma13030513
APA StyleSilva, F., Paulo, A., Pallier, A., Même, S., Tóth, É., Gano, L., Marques, F., Geraldes, C. F. G. C., Castro, M. M. C. A., Cardoso, A. M., Jurado, A. S., López-Larrubia, P., Lacerda, S., & Cabral Campello, M. P. (2020). Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials, 13(3), 513. https://doi.org/10.3390/ma13030513