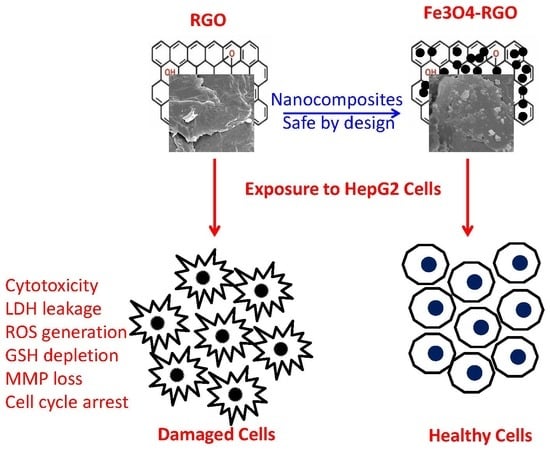
Investigation of Cytotoxicity, Apoptosis, and Oxidative Stress Response of Fe3O4-RGO Nanocomposites in Human Liver HepG2 cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of RGO, Fe3O4 NPs and Fe3O4-RGO Nanocomposites
2.2. Characterization
2.3. Cell Culture and Exposure of NPs and Nanocomposites
2.4. Biochemical Studies
2.5. Statistics
3. Results and Discussion
3.1. Characterization of RGO, Fe3O4 NPs, and Fe3O4-RGO Nanocomposites
3.2. Cytotoxicity of Fe3O4 NPs, RGO and Fe3O4-RGO Nanocomposites
3.3. Apoptotic Response of RGO and Fe3O4-RGO Nanocomposites
3.4. Oxidative Stress Response of RGO and Fe3O4-RGO Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Y.; Ren, J.; Qu, X. A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials 2011, 32, 2930–2937. [Google Scholar] [CrossRef]
- Liang, C.; Zhai, T.; Wang, W.; Chen, J.; Zhao, W.; Lu, X.; Tong, Y. Fe3O4/reduced graphene oxide with enhanced electrochemical performance towards lithium storage. J. Mater. Chem. A 2014, 2, 7214–7220. [Google Scholar] [CrossRef]
- He, F.A.; Fan, J.T.; Song, F.; Zhang, L.M.; Chan, H.W. Fabrication of hybrids based on graphene and metal nanoparticles by in situ and self-assembled methods. Nanoscale 2011, 3, 1182–1188. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X. Graphene–inorganic nanocomposites. RSC Adv. 2012, 2, 64–98. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R.; Singh, S.P.; Rengan, A.K.; Szunerits, S.; Boukherroub, R. Ag and Au nanoparticles/reduced graphene oxide composite materials: Synthesis and application in diagnostics and therapeutics. Adv. Colloid Interface Sci. 2019, 271, 101991. [Google Scholar] [CrossRef]
- How, G.T.; Pandikumar, A.; Ming, H.N.; Ngee, L.H. Highly exposed {001} facets of titanium dioxide modified with reduced graphene oxide for dopamine sensing. Sci. Rep. 2014, 4, 5044. [Google Scholar] [CrossRef] [Green Version]
- Madhuvilakku, R.; Alagar, S.; Mariappan, R.; Piraman, S. Green one-pot synthesis of flowers-like Fe3O4/rGO hybrid nanocomposites for effective electrochemical detection of riboflavin and low-cost supercapacitor applications. Sens. Actuators B 2017, 253, 879–892. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.H.; Kim, J.H. Graphene Oxide–Platinum Nanoparticle Nanocomposites: A Suitable Biocompatible Therapeutic Agent for Prostate Cancer. Polymers 2019, 11, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Gurunathan, S.; Kim, J.H. Graphene Oxide-Silver Nanocomposite Enhances Cytotoxic and Apoptotic Potential of Salinomycin in Human Ovarian Cancer Stem Cells (OvCSCs): A Novel Approach for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Ding, J.; Xue, J. A new family of biocompatible and stable magnetic nanoparticles: Silica cross-linked pluronic F127 micelles loaded with iron oxides. New J. Chem. 2009, 33, 88. [Google Scholar] [CrossRef]
- Gupta, J.; Bhargava, P.; Bahadur, D. Methotrexate conjugated magnetic nanoparticle for targeted drug delivery and thermal therapy. J. Appl. Phys. 2014, 115, 17B516. [Google Scholar] [CrossRef]
- Maeng, J.H.; Lee, D.H.; Jung, K.H.; Bae, Y.H.; Park, I.S.; Jeong, S.; Jeon, Y.S.; Shim, C.K.; Kim, W.; Kim, J.; et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef]
- Ying, X.Y.; Du, Y.Z.; Hong, L.H.; Yuan, H.; Hu, F.Q. Magnetic lipid nanoparticles loading doxorubicin for intracellular delivery: Preparation and characteristics. J. Magn. Magn. Mater. 2011, 323, 1088–1093. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Obaidat, I.M.; Kamzin, A.S.; Latiyan, S.; Jain, S.; Kumar, H.; Srivastava, C.; Alaabed, S.; Issa, B. Synthesis of Graphene Oxide-Fe3O4 Based Nanocomposites Using the Mechanochemical Method and in Vitro Magnetic Hyperthermia. Int. J. Mol. Sci. 2019, 20, 3368. [Google Scholar] [CrossRef] [Green Version]
- Gupta, J.; Prakash, A.; Jaiswal, M.K.; Agarrwal, A.; Bahadur, D. Superparamagnetic iron oxide-reduced graphene oxide nanohybrid-a vehicle for targeted drug delivery and hyperthermia treatment of cancer. J. Magn. Magn. Mater. 2018, 448, 332–338. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef]
- Chen, P.; Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol. Environ. Saf. 2019, 171, 337–346. [Google Scholar] [CrossRef]
- Liu, L.; Dai, H.; Wu, Y.; Li, B.; Yi, J.; Xu, C.; Wu, X. In vitro and in vivo mechanism of hepatocellular carcinoma inhibition by β-TCP nanoparticles. Int. J. Nanomed. 2019, 14, 3491–3502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sydlik, S.A.; Jhunjhunwala, S.; Webber, M.J.; Anderson, D.G.; Langer, R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano 2015, 9, 3866–3874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonca, M.C.P.; Soares, E.S.; de Jesus, M.B.; Ceragioli, H.J.; Irazusta, S.P.; Batista, A.G.; Vinolo, M.A.R.; Júnior, M.R.M.; Cruz-Höfling, M.A. Reduced graphene oxide: Nanotoxicological profile in rats. J. Nanobiotechnol. 2016, 14, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.J.; Li, P.; Zhao, X.L.; Liu, Z.Y.; Fan, Q.H.; Li, Z.; Li, J.X.; Wang, D. Distinct interface behaviors of Ni(II) on graphene oxide and oxidized carbon nanotubes triggered by deferent topological aggregations. Nanoscale 2018, 10, 1383–1393. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.S.; Alrokayan, S.A. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 2011, 283, 101–108. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Khan, M.A.; Alrokayan, S.A. Comparative cytotoxic response of nickel ferrite nanoparticles in human liver HepG2 and breast MFC-7 cancer cells. Chemosphere 2015, 135, 278–288. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.I. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Khan, W.; Ahamed, M.; Alhazaa, A.N. Investigation on the structure and physical properties of Fe3O4/RGO nanocomposites and their photocatalytic application. Mat. Sci. Semicond. Process. 2019, 99, 44–53. [Google Scholar] [CrossRef]
- Zhu, S.; Fan, L.; Lu, Y. Highly uniform Fe3O4 nanoparticle–rGO composites as anode materials for high performance lithium-ion batteries. RSC Adv. 2017, 7, 54939–54946. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, J.S.; Pendashteh, A.; Palma, J.; Anderson, M.; Marcilla, R. Anchored Fe3O4 Nanoparticles on rGO Nanosheets as High-Power Negative Electrodes for Aqueous Batteries. ChemElectroChem 2017, 4, 1295–1305. [Google Scholar] [CrossRef]
- Zhou, L.; Deng, H.; Wan, J.; Shi, J.; Su, T. A solvothermal method to produce RGO-Fe3O4 hybrid composite for fast chromium removal from aqueous solution. Appl. Surf. Sci. 2013, 283, 1024–1031. [Google Scholar] [CrossRef]
- Chen, D.; Tang, Q.; Li, X.; Zhou, X.; Zang, J.; Xue, W.; Xiang, J.; Guo, C. Biocompatibility of magnetic Fe3O4 nanoparticles and their cytotoxic effect on MCF-7 cells. Int. J. Nanomed. 2012, 7, 4973–4982. [Google Scholar] [CrossRef] [Green Version]
- Ankamwar, B.; Lai, T.C.; Huang, J.H.; Liu, R.S.; Hsiao, M.; Chen, C.H.; Hwu, Y.K. Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology 2010, 21, 7. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Cha, R.; Ran, B.; Mou, K.; Wang, H.; Xie, Q.; Sun, J.; Jiang, X. The biocompatibility evaluation of iron oxide nanoparticles synthesized by a one pot process for intravenous iron supply. RSC Adv. 2016, 6, 14329–14334. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Singh, V.; Joung, D.; Dowding, J.M.; Reid, D.; Anderson, J.; Zhai, L.; Khondaker, S.I.; Self, W.T.; et al. Oxygenated functional group density on graphene oxide: Its effect on cell toxicity. Part. Part. Syst. Charact. 2013, 30, 148–157. [Google Scholar] [CrossRef]
- Mittal, S.; Kumar, V.; Dhiman, N.; Chauhan, L.K.; Pasricha, R.; Pandey, A.K. Physico-chemical properties based di_erential toxicity of graphene oxide/reduced graphene oxide in human lung cells mediated through oxidative stress. Sci. Rep. 2016, 6, 39548. [Google Scholar] [CrossRef]
- Zhang, B.; Lung, P.S.; Zhao, S.; Chu, Z.; Chrzanowski, W.; Li, Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Sci. Rep. 2017, 7, 7315. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, C.; She, Y.; Zhang, H.; Song, M.; Han, Y.; Li, Y.; Zhu, Y. Cytoprotective e_ect of deferiprone against aluminum chloride-induced oxidative stress and apoptosis in lymphocytes. Toxicol. Lett. 2018, 285, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Di_erential cytotoxicity of deferent sizes of Graphene oxide nanoparticles in leydig (TM3) and sertoli (TM4) cells. Nanomaterials 2019, 9, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, S.; Wang, M.; Liang, J.; Zhao, X.; Fan, Q.; Geng, R.; Luo, D.; Li, Z.; Zhang, L. E_ects of morphology regulated by Pb2+ on graphene oxide cytotoxicity: Spectroscopic and in vitro investigations. Mater. Chem. Phys. 2020, 239, 122016. [Google Scholar] [CrossRef]
- Ali, D.; Alarifi, S.; Alkahtani, S.; Almeer, R.S. Silver-doped graphene oxide nanocomposite triggers cytotoxicity and apoptosis in human hepatic normal and carcinoma cells. Int. J. Nanomed. 2018, 13, 5685–5699. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Kong, L.; Wu, T.; Zhang, T.; Tang, M.; et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020, 256, 113430. [Google Scholar] [CrossRef]
- Rees, P.; Wills, J.W.; Brown, M.R.; Barnes, C.M.; Summers, H.D. The origin of heterogeneous nanoparticle uptake by cells. Nat. Commun. 2019, 10, 2341. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Alhadlaq, H.A.; Alrokayan, S.A. Assessment of the lung toxicity of copper oxide nanoparticles: Current status. Nanomedicine 2015, 10, 2365–2377. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Carocho, M.; Ferreira, I.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nano-level. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarosz, A.; Skoda, M.; Dudek, I.; Szukiewicz, D. Oxidative Stress and Mitochondrial Activation as the Main Mechanisms Underlying Graphene Toxicity against Human Cancer Cells. Oxidative Med. Cell. Longev. 2016, 2016, 5851035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheen, F.; Aziz, M.H.; Fatima, M.; Khan, M.A.; Ahmed, F.; Ahmad, R.; Ahmad, M.A.; Alkhuraiji, T.S.; Akram, M.W.; Raza, R.; et al. In Vitro cytotoxicity and morphological assessments of GO-ZnO against the MCF-7 Cells: Determination of singlet oxygen by chemical trapping. Nanomaterials 2018, 8, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, R.; Panayiotidis, M.I.; Cidlowski, J.A. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J. Biol. Chem. 2007, 282, 30452–30465. [Google Scholar] [CrossRef] [Green Version]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahamed, M.; Akhtar, M.J.; Khan, M.A.M. Investigation of Cytotoxicity, Apoptosis, and Oxidative Stress Response of Fe3O4-RGO Nanocomposites in Human Liver HepG2 cells. Materials 2020, 13, 660. https://doi.org/10.3390/ma13030660
Ahamed M, Akhtar MJ, Khan MAM. Investigation of Cytotoxicity, Apoptosis, and Oxidative Stress Response of Fe3O4-RGO Nanocomposites in Human Liver HepG2 cells. Materials. 2020; 13(3):660. https://doi.org/10.3390/ma13030660
Chicago/Turabian StyleAhamed, Maqusood, Mohd Javed Akhtar, and M. A. Majeed Khan. 2020. "Investigation of Cytotoxicity, Apoptosis, and Oxidative Stress Response of Fe3O4-RGO Nanocomposites in Human Liver HepG2 cells" Materials 13, no. 3: 660. https://doi.org/10.3390/ma13030660
APA StyleAhamed, M., Akhtar, M. J., & Khan, M. A. M. (2020). Investigation of Cytotoxicity, Apoptosis, and Oxidative Stress Response of Fe3O4-RGO Nanocomposites in Human Liver HepG2 cells. Materials, 13(3), 660. https://doi.org/10.3390/ma13030660