Experimental Study on Water Recovery from Flue Gas Using Macroporous Ceramic Membrane
Abstract
:1. Introduction
2. Experiment and Calculation Method
2.1. Structural Characterization and Water Recovery Mechanism
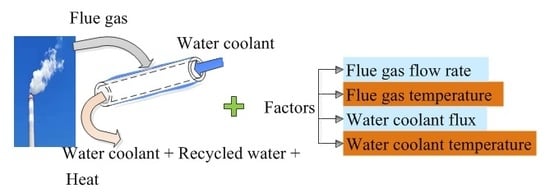
2.2. Experimental System
2.3. Recovery Performance Calculation Method
2.4. Uncertainty Analysis
3. Results and Discussion
3.1. Flue Gas Flow Rate
3.2. Flue Gas Temperature
3.3. Water Coolant Flux
3.4. Water Coolant Temperature
3.5. Comparison of Different Research Results
4. Conclusions
- With the flue gas flow rate increasing, the amount of recycled water and recovered heat increased linearly, while the recycled water rate and heat recovery rate dropped.
- The amount of recycled water, recycled water rate, and recovered heat increased with the increase in flue gas temperature. The growth trend of recovered heat was slower than that of the maximum recoverable heat, which resulted in a decrease in heat recovery rate.
- Along with water coolant temperature growth, the amount of recycled water, recycled water rate, recovered heat, and heat recovery rate decreased. A higher temperature resulted in a more serious deterioration of water vapor condensation. When the water coolant temperature exceeded 30 °C, the amount of recycled water dropped sharply.
- Under the experimental conditions, the maximum amounts of recycled water, recovered heat, and total heat transfer coefficient were 2.93 kg/(m2·h), 3.63 kW/m2, and 224.3 W/(m2·K), respectively.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Liu, J.; Yu, T.; Xu, Z.; Hong, Y.; Gerbensleenes, P.W.; Vliet, M.T.H.V.; Yan, J. China’s coal-fired power plants impose pressure on water resources. J. Clean. Prod. 2017, 161, 1171–1179. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, C.; Cai, W.; Kummu, M.; Varis, O. The vulnerability of thermoelectric power generation to water scarcity in China: Current status and future scenarios for power planning and climate change. Appl. Energy 2016, 171, 444–455. [Google Scholar] [CrossRef]
- China Electricity Council. A List of the Country’s Electricity Industry Statistics from January to September in 2019; CEIC: Beijing, China, 2019. [Google Scholar]
- Chen, H.; Zhou, Y.; Cao, S.; Li, X.; Su, X.; An, L.; Gao, D. Heat exchange and water recovery experiments of flue gas with using nanoporous ceramic membranes. Appl. Therm. Eng. 2017, 110, 686–694. [Google Scholar] [CrossRef]
- Kim, J.F.; Park, A.; Kim, S.; Lee, P.; Cho, Y.H.; Park, H.; Nam, S.E.; You, I.P. Harnessing clean water from power plant emissions using membrane condenser technology. ACS Sustain. Chem. Eng. 2018, 6, 6425–6433. [Google Scholar] [CrossRef]
- Liao, X.; Hall, W.J.; Eyre, N. Water use in China’s thermoelectric power sector. Glob. Environ. Chang. 2016, 41, 142–152. [Google Scholar] [CrossRef]
- Xiong, Y.; Tan, H.; Wang, Y.; Xu, W.; Mikulčić, H.; Duić, N. Pilot-scale study on water and latent heat recovery from flue gas using fluorine plastic heat exchangers. J. Clean. Prod. 2017, 161, 1416–1422. [Google Scholar] [CrossRef]
- Chen, Q.; Finney, K.; Li, H.; Zhang, X.; Zhou, J.; Sharifi, V.; Swithenbank, J. Condensing boiler applications in the process industry. Appl. Energy 2012, 89, 30–36. [Google Scholar] [CrossRef]
- Ito, A. Dehumidification of air by a hygroscopic liquid membrane supported on surface of a hydrophobic microporous membrane. J. Membr. Sci. 2000, 175, 35–42. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Qu, K.Y.; Jiang, Y. Experimental study on mass transfer performances of cross flow dehumidifier using liquid desiccant. Energy Convers. Manag. 2006, 47, 2682–2692. [Google Scholar] [CrossRef]
- Macedonio, F.; Brunetti, A.; Barbieri, G.; Drioli, E. Membrane condenser as a new technology for water recovery from humidified “Waste” gaseous streams. Ind. Eng. Chem. Res. 2013, 52, 1160–1167. [Google Scholar] [CrossRef]
- Asfand, F.; Bourouis, M. A review of membrane contactors applied in absorption refrigeration systems. Renew. Sustain. Energy Rev. 2015, 45, 173–191. [Google Scholar] [CrossRef]
- Sijbesma, H.; Nymeijer, K.; Marwijk, R.V.; Heijboer, R.; Potreck, J.; Wessling, M. Flue gas dehydration using polymer membranes. J. Membr. Sci. 2008, 313, 263–276. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Zhang, H.; Chen, H. Moisture and latent heat recovery from flue gas by nonporous organic membranes. J. Clean. Prod. 2019, 225, 1065–1078. [Google Scholar] [CrossRef]
- Brunetti, A.; Santoro, S.; Macedonio, F.; Figoli, A.; Drioli, E.; Barbieri, G. Waste gaseous streams: From environmental issue to source of water by using membrane condensers. Clean.-Soil Air Water 2014, 42, 1145–1153. [Google Scholar] [CrossRef]
- Wang, D. Transport Membrane Condenser for Water and Energy Recovery from Power Plant Flue Gas; Technical Reports; Office of Scientific & Technical Information: Des Plaines, IL, USA, 2012. [Google Scholar]
- Wang, D.; Bao, A.; Kunc, W.; Liss, W. Coal power plant flue gas waste heat and water recovery. Appl. Energy 2012, 91, 341–348. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, M.; Zhao, S.; Yuan, W.; Huang, S. Entropy generation analysis of heat and water recovery from flue gas by transport membrane condenser. Energy 2019, 174, 835–847. [Google Scholar] [CrossRef]
- Soleimanikutanaei, S.; Lin, C.X.; Wang, D. Modeling and simulation of cross-flow transport membrane condenser heat exchangers. Int. Commun. Heat Mass 2018, 95, 92–97. [Google Scholar] [CrossRef]
- Soleimanikutanaei, S.; Lin, C.X.; Wang, D. Numerical modeling and analysis of Transport Membrane Condensers for waste heat and water recovery from flue gas. Int. J. Therm. Sci. 2019, 136, 96–106. [Google Scholar] [CrossRef]
- Wang, T.; Yue, M.; Qi, H.; Feron, P.H.M.; Zhao, S. Transport membrane condenser for water and heat recovery from gaseous streams: Performance evaluation. J. Membr. Sci. 2015, 484, 10–17. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Su, X.; Cao, S. Experimental study of water recovery from flue gas using hollow micro-nano porous ceramic composite membranes. J. Ind. Eng. Chem. 2018, 57, 349–355. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, H.; Xie, T.; Wang, B. Effect of mass transfer on heat transfer of microporous ceramic membranes for water recovery. Int. J. Heat Mass Transf. 2017, 112, 643–648. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Zhang, H.; Wang, L. The investigation of desulphurisation and water recovery from flue gas using ceramic composite membrane. Int. J. Energy Res. 2019, 43, 1747–1759. [Google Scholar] [CrossRef]
- Yue, M.; Zhao, S.; Feron, P.H.M.; Hong, Q. Multichannel tubular ceramic membrane for water and heat recovery from waste gas streams. Ind. Eng. Chem. Res. 2016, 55, 2615–2622. [Google Scholar] [CrossRef]
- Hu, H.; Tang, G.H.; Niu, D. Wettability modified nanoporous ceramic membrane for simultaneous residual heat and condensate recovery. Sci. Rep.-UK 2016, 6, 27274. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhou, J.E.; Lin, B.; Wang, Y.; Wang, S.; Miao, L.; Lang, Y.; Liu, X.; Meng, G. Reaction-sintered porous mineral-based mullite ceramic membrane supports made from recycled materials. J. Hazard. Mater. 2009, 172, 180–186. [Google Scholar] [CrossRef]
- Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from inexpensive raw materials by extrusion method and its performance in microfiltration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; Sánchez, E. Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, S.; Wang, D.K.; Wei, Y.; Qi, H.; Wu, T.; Feron, P.H.M. Simultaneous heat and water recovery from flue gas by membrane condensation: Experimental investigation. Appl. Therm. Eng. 2017, 113, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Richter, H.; Voss, H.; Kaltenborn, N.; Kamnitz, S.; Feldhoff, A.; Caro, J.; Roitsch, S.; Voigt, I.; Wollbrink, A. High-flux carbon molecular sieve membranes for gas separation. Angew. Chem. 2017, 129, 7868–7871. [Google Scholar] [CrossRef]
- Uhlhorn, R.J.R.; Keizer, K.; Burggraaf, A.J. Gas transport and separation with ceramic membranes. Part I. Multilayer diffusion and capillary condensation. J. Membr. Sci. 1992, 66, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface 2011, 169, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Ruthven, D.M.; Desisto, W.J.; Higgins, S. Diffusion in a mesoporous silica membrane: Validity of the Knudsen diffusion model. Chem. Eng. Sci. 2009, 64, 3201–3203. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Zhang, H.; Chen, H. Moisture recovery from gas-fired boiler exhaust using membrane module array. J. Clean. Prod. 2019, 231, 1110–1121. [Google Scholar] [CrossRef]
- Yang, S.; Tao, W. Heat Transfer; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Ma, S.; Chai, J.; Jiao, K.; Ma, L.; Zhu, S.; Wu, K. Environmental influence and countermeasures for high humidity flue gas discharging from power plants. Renew. Sustain. Energy Rev. 2017, 73, 225–235. [Google Scholar]










| Item | Unit | Value |
|---|---|---|
| Flue gas flow rate | kg/s | 6.25 × 10−5 to 3.125 × 10−4 |
| Flue gas temperature | °C | 40; 50; 60 |
| Relative humidity | % | 100 |
| Water coolant flux | kg/s | 8.32 × 10−3 to 3.327 × 10−2 |
| Water coolant temperature | °C | 15–35 |
| Experimental Apparatus | Model | Parameters | Precision | Manufacturer |
|---|---|---|---|---|
| Gas flow controller | D07-9E | 30 SLM; Max pressure:3 MPa | ±2% | Beijing Sevenstar, Beijing, China |
| Electric thermostatic water tank | HH.W21.600 | Rated power: 750 W ± 10%; | ±0.5 °C | Shanghai shuli, Shanghai, China |
| Temperature and humidity transmitter | TH-21E | Temperature range: −40 to 125 °C Relative humidity range: 0–100% | ≤±0.2 °C ≤±2% | Guangzhou Anymetre, Guangzhou, China |
| Eight-loop digital display device | HT-MK807-01-23-KL | - | 0.5% FS | Hantang Precision Instrument, Wuxi, China |
| Thermocouple | PT100 | −50 to 200 °C | A Class | Hangzhou Sinomeasure, Hangzhou, China |
| Miniature electric diaphragm pump | PLD-1205 | Maximum flow rate: 3.2 L/min | - | Shijiazhuang Pulandi, Shijiazhuang, China |
| Flowmeter | LZT-M15 | Range: 0.2–2.0 L/min | ≤±4% | Vakada, Suzhou, China |
| Reference | Pore Size | Membrane Area (m2) | Coating | Component | Water Flux kg/(m2·h) | Experimental Conditions |
|---|---|---|---|---|---|---|
| [4] | 20 nm | 0.025 | Inner coating | N2/water vapor | 5.7 | Inlet gas temperature and flow rate were 60 °C and 14 L/min, respectively; coolant water temperature and flow rate were 16 °C and 2 L/min, respectively |
| [21] | 6–8 nm | 0.0021 | Inner coating | Air/water vapor | 15.8 | Inlet gas temperature and flow rate were 75 °C and 4 L/min, respectively; coolant water temperature and flow rate were 33 °C and 5 L/h, respectively |
| [30] | 7 nm | 0.0021 | Inner coating | Air/water vapor | 4.5 | Inlet gas temperature and flow rate were 100 °C and 6.7 L/min, respectively; coolant water flow rate was 3.3 L/h |
| [35] | 1 μm | 0.7 | Outer coating | Gas-fired boiler flue gas | 15.8 | Inlet gas temperature and flow rate were 46 °C and 1600 m3/h, respectively; coolant water temperature and flow rate were 23 °C and 1150 L/h, respectively |
| This paper | 1 μm | 0.0294 | Outer coating | N2/water vapor | 2.6 | Inlet gas temperature and flow rate were 50 °C and 15 L/min, respectively; coolant water temperature and flow rate were 20 °C and 1 L/min, respectively |
| Ref | Flue gas Flow Rate | Flue Gas Temperature | Coolant Water Flow Rate | Coolant Water Temperature | |
|---|---|---|---|---|---|
| [4] | Water flux | Increased linearly | Increased exponentially | Changed little | Decreased parabolically |
| Heat flux | Increased linearly | Increased exponentially | Increased | - | |
| THTC | - | - | - | - | |
| [21] | Water flux | Increased linearly | Increased exponentially | Increased slightly | - |
| Heat flux | Increased exponentially | Increased exponentially | increased linearly | - | |
| THTC | - | - | - | - | |
| [30] | Water flux | Decreased linearly | Increased linearly | Increased linearly | Decreased linearly |
| Heat flux | Decreased linearly | Increased linearly | Increased linearly | Decreased linearly | |
| THTC | Decreased parabolically | Decreased lightly | Increased linearly | Decreased linearly | |
| [35] | Water flux | Increased linearly | Increased linearly | Increased linearly | Decreased linearly |
| Heat flux | Increased linearly | Increased linearly | Increased linearly | Decreased linearly | |
| THTC | Increased linearly | Changed little | Increased linearly | Decreased linearly | |
| This paper | Water flux | Increased linearly | Increased exponentially | Increased lightly | Decreased lightly |
| Heat flux | Increased linearly | Increased exponentially | Increased linearly | Decreased linearly | |
| THTC | Increased linearly | Increased exponentially | Increased linearly | Increased |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Zhang, H.; Chen, H. Experimental Study on Water Recovery from Flue Gas Using Macroporous Ceramic Membrane. Materials 2020, 13, 804. https://doi.org/10.3390/ma13030804
Cheng C, Zhang H, Chen H. Experimental Study on Water Recovery from Flue Gas Using Macroporous Ceramic Membrane. Materials. 2020; 13(3):804. https://doi.org/10.3390/ma13030804
Chicago/Turabian StyleCheng, Chao, Heng Zhang, and Haiping Chen. 2020. "Experimental Study on Water Recovery from Flue Gas Using Macroporous Ceramic Membrane" Materials 13, no. 3: 804. https://doi.org/10.3390/ma13030804
APA StyleCheng, C., Zhang, H., & Chen, H. (2020). Experimental Study on Water Recovery from Flue Gas Using Macroporous Ceramic Membrane. Materials, 13(3), 804. https://doi.org/10.3390/ma13030804