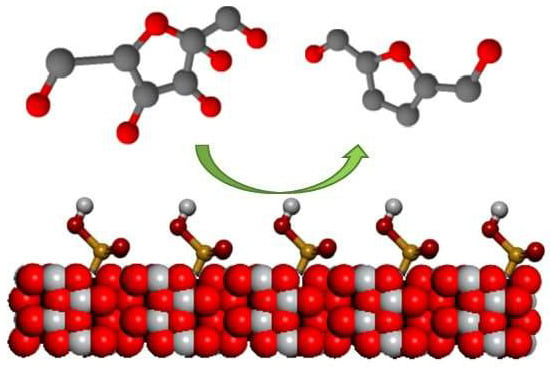
Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Preparation
2.3. Catalyst Characterizations
2.4. Hydrothermal Fructose Dehydration
3. Result and Discussion
3.1. Structural Characterization
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ventura, M.; Domine, M.E.; Chavez-Sifontes, M. Catalytic Processes For Lignin Valorization into Fuels and Chemicals (Aromatics). Curr. Catal. 2019, 8, 20–40. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Converting carbohydrates to bulk chemicals and fine chemicals over heterogeneous catalysts. Green Chem. 2011, 13, 520–540. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2, 5-dimethylfuran. Renew. Sustain. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on its Manufacture. Starch Stärke 1990, 42, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Fachri, B.A.; Abdilla, R.M.; Bovenkamp, H.H.V.D.; Rasrendra, C.B.; Heeres, H.J. Experimental and Kinetic Modeling Studies on the Sulfuric Acid Catalyzed Conversion of d-Fructose to 5-Hydroxymethylfurfural and Levulinic Acid in Water. ACS Sustain. Chem. Eng. 2015, 3, 3024–3034. [Google Scholar] [CrossRef]
- Zhang, J.; Weitz, E. An in Situ NMR Study of the Mechanism for the Catalytic Conversion of Fructose to 5-Hydroxymethylfurfural and then to Levulinic Acid Using 13C Labeled d-Fructose. ACS Catal. 2012, 2, 1211–1218. [Google Scholar] [CrossRef]
- Kimura, H.; Nakahara, M.; Matubayasi, N. Solvent Effect on Pathways and Mechanisms for d-Fructose Conversion to 5-Hydroxymethyl-2-furaldehyde: In Situ 13C NMR Study. J. Phys. Chem. A 2013, 117, 2102–2113. [Google Scholar] [CrossRef]
- Kılıç, E.; Yılmaz, S. Fructose Dehydration to 5-Hydroxymethylfurfural over Sulfated TiO2–SiO2, Ti-SBA-15, ZrO2, SiO2, and Activated Carbon Catalysts. Ind. Eng. Chem. Res. 2015, 54, 5220–5225. [Google Scholar] [CrossRef] [Green Version]
- Román-Leshkov, Y.; Dumesic, J.A. Solvent Effects on Fructose Dehydration to 5-Hydroxymethylfurfural in Biphasic Systems Saturated with Inorganic Salts. Top. Catal. 2009, 52, 297–303. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P.; Rivalier, P.; Ros, P.; Avignon, G. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Appl. Catal. A 1996, 145, 211–224. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; van der Schaaf, J.; Schouten, J.C.; Nijhuis, T.A. Fructose Dehydration to 5-Hydroxymethylfurfural over Solid Acid Catalysts in a Biphasic System. ChemSusChem 2012, 5, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Kourieh, R.; Rakic, V.; Bennici, S.; Auroux, A. Relation between surface acidity and reactivity in fructose conversion into 5-HMF using tungstated zirconia catalysts. Catal. Commun. 2013, 30, 5–13. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Z.; Yi, X.; Wang, S.; Li, J.; Wang, X.; Jiang, Z. A water-tolerant C16H3PW11CrO39 catalyst for the efficient conversion of monosaccharides into 5-hydroxymethylfurfural in a micellar system. RSC Adv. 2013, 3, 23051–23056. [Google Scholar] [CrossRef]
- Jadhav, A.H.; Kim, H.; Hwang, I.T. An efficient and heterogeneous recyclable silicotungstic acid with modified acid sites as a catalyst for conversion of fructose and sucrose into 5-hydroxymethylfurfural in superheated water. Bioresour. Technol. 2013, 132, 342–350. [Google Scholar] [CrossRef]
- Tian, C.; Oyola, Y.; Nelson, K.M.; Chai, S.-H.; Zhu, X.; Bauer, J.C.; Janke, C.J.; Brown, S.; Guo, Y.; Dai, S. A renewable HSO3/H2PO3-grafted polyethylene fiber catalyst: An efficient heterogeneous catalyst for the synthesis of 5-hydroxymethylfurfural from fructose in water. RSC Adv. 2013, 3, 21242–21246. [Google Scholar] [CrossRef]
- Huang, Y.; Chao, P.-Y.; Cheng, T.-Y.; Ho, Y.; Lin, C.-T.; Hsu, H.-Y.; Wong, J.-J.; Tsai, T.-C. Design of sulfonated mesoporous silica catalyst for fructose dehydration guided by difructose anhydride intermediate incorporated reaction network. Chem. Eng. J. 2016, 283, 778–788. [Google Scholar] [CrossRef]
- Date, N.S.; La Parola, V.; Rode, C.V.; Testa, M.L. Ti-Doped Pd-Au Catalysts for One-Pot Hydrogenation and Ring Opening of Furfural. Catalysts 2018, 8, 252. [Google Scholar] [CrossRef] [Green Version]
- Beejapur, H.A.; La Parola, V.; Liotta, L.F.; Testa, M.L. Glycerol Acetylation over Organic-Inorganic Sulfonic or Phosphonic Silica Catalysts. ChemistrySelect 2017, 2, 4934–4941. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Sanz, R.; Pizarro, P. Preparation of bimodal micro–mesoporous TiO2 with tailored crystalline properties. Chem. Commun. 2004, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- FIZ Karlsruhe. Inorganic Crystal Structure Database (ICSD); FIZ Karlsruhe: Karlsruhe, Germany, 2019. [Google Scholar]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase Modifiers Promote Efficient Production of Hydroxymethylfurfural from Fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Catalyst | BET | XPS | Acidity (mmol H+g−1) | |||
|---|---|---|---|---|---|---|
| SSA (m2g−1) | Vp (cm3g−1) | S/Si | S/Ti | Si/Ti | ||
| TiO2 | 45 | 0.10 | 0.08 | |||
| TiO2 P25 | 56 | 0.24 | 0.08 | |||
| TiO2-Pr-SO3H | 41 | 0.09 | 0.35 | 0.19 | 0.53 | 0.16 |
| TiO2-Pr-SO3H P25 | 39 | 0.30 | 0.73 | 0.58 | 0.80 | 0.73 |
| TiO2-SO3H | 37 | 0.07 | 0.17 | 0.30 | ||
| TiO2-SO3H P25 | 35 | 0.38 | 0.43 | 1.60 | ||
| Time (h) | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| 1 | 82 | 53 | 43 |
| 2 | 89 | 51 | 45 |
| 3 | 99 | 50 | 50 |
| (*) 3 | 99 | 66 | 65 |
| Catalyst Amount (mg) | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| (*) 6.0 | 9 | 78 | 7 |
| 6.0 | 25 | 48 | 12 |
| 18.0 | 37 | 59 | 22 |
| 36.0 | 42 | 49 | 20 |
| Catalyst Amount (mg) | Conversion (%) | Selectivity (%) | Yield (%) |
|---|---|---|---|
| 6.0 | 14 | 31 | 4 |
| 18.0 | 13 | 71 | 9 |
| (*) 18.0 | 29 | 55 | 16 |
| 36.0 | 10 | 88 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testa, M.L.; Miroddi, G.; Russo, M.; La Parola, V.; Marcì, G. Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials 2020, 13, 1178. https://doi.org/10.3390/ma13051178
Testa ML, Miroddi G, Russo M, La Parola V, Marcì G. Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials. 2020; 13(5):1178. https://doi.org/10.3390/ma13051178
Chicago/Turabian StyleTesta, Maria Luisa, Gianmarco Miroddi, Marco Russo, Valeria La Parola, and Giuseppe Marcì. 2020. "Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts" Materials 13, no. 5: 1178. https://doi.org/10.3390/ma13051178
APA StyleTesta, M. L., Miroddi, G., Russo, M., La Parola, V., & Marcì, G. (2020). Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials, 13(5), 1178. https://doi.org/10.3390/ma13051178