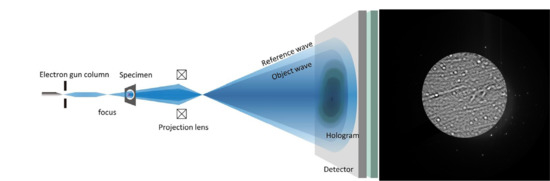
In-Line Holography in Transmission Electron Microscopy for the Atomic Resolution Imaging of Single Particle of Radiation-Sensitive Matter
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Vincamine
3.2. Caffeine/Glutaric Acid Co-Crystals
3.3. Creatinine-Ferrihydrite Nanoparticles
4. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Van Aert, S.; Batenburg, K.J.; Rossell, M.D.; Erni, R.; Van Tandeloo, G. Three-dimensional atomic imaging of crystalline nanoparticles. Nature 2011, 470, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.C.H. Experimental High-Resolution Electron Microscopy, 2nd ed.; Oxford University Press Inc.: New York, NY, USA, 1988; ISBN 0-19-505405-9. [Google Scholar]
- Wisedchaisri, G.; Reichow, S.L.; Gonen, T. Advances in structural and functional analysis of membrane proteins by electron crystallography. Structure 2011, 19, 1381–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasa, D.; Perissutti, B.; Cepek, C.; Bhardwaj, S.; Carlino, E.; Grassi, M.; Invernizzi, S.; Voinovich, D. Drug salt formation via mechanochemistry: The case study of vincamine. Mol. Pharm. 2013, 10, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Hasa, D.; Carlino, E.; Jones, W. Polymer-assisted grinding, a versatile method for polymorph control of cocrystallization. Cryst. Growth Des. 2016, 16, 1772–1779. [Google Scholar] [CrossRef]
- Egerton, R.F. Control of radiation damage in the TEM. Ultramicroscopy 2013, 127, 100–108. [Google Scholar] [CrossRef]
- Malac, M.; Beleggia, M.; Egerton, R.; Zhu, Y. Cs corrected bright field TEM imaging of radiation sensitive materials. Microsc. Microanal. 2005, 2, 2150–2151. [Google Scholar] [CrossRef] [Green Version]
- Malac, M.; Beleggia, M.; Taniguchi, Y.; Egerton, R.F.; Zhu, Y. Low-dose performance of parallel-beam nanodiffraction. Ultramicroscopy 2008, 109, 14–21. [Google Scholar] [CrossRef]
- Egerton, R.F. Outrun radiation damage with electrons? Adv. Struct. Chem. Imaging 2015, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef]
- Knauer, W. Boersch effect in electron-optical instruments. J. Vac. Sci. Technol. 1979, 16, 1676–1679. [Google Scholar] [CrossRef]
- Egerton, R.F.; McLeod, R.; Wang, F.; Malac, M. Basic questions related to electron-induced sputtering in the TEM. Ultramicroscopy 2010, 110, 991–997. [Google Scholar] [CrossRef]
- Vereczkey, L. Pharmacokinetics and metabolism of vincamine and related compounds. Eur. J. Drug Metab. Pharmacokinet. 1985, 10, 89–103. [Google Scholar] [CrossRef]
- Karpati, E.; Biro, K.; Kukorelli, T. Investigation of vasoactive agents with indole skeletons at Richter Ltd. Acta Pharm. Hung. 2002, 72, 25–36. [Google Scholar]
- Vas, A.; Gulyas, B. Eburnamine derivatives and the brain. Med. Res. Rev. 2005, 25, 737–757. [Google Scholar] [CrossRef]
- Leijten, Z.J.W.A.; Keizer, A.D.A.; de With, G.; Friedrich, H. Quantitative analysis of electron Beam damage in organic thin films. J. Phys. Chem. C 2017, 121, 10552–10561. [Google Scholar] [CrossRef]
- Egerton, R.F.; Li, P.; Malac, M. Radiation damage in the TEM and SEM. Micron 2004, 35, 399–409. [Google Scholar] [CrossRef]
- Hobbs, L.W. Radiation effects in analysis by TEM. In Introduction to Analytical Electron Microscopy; Hren, J.J., Goldstein, J.I., Joy, D.C., Eds.; Plenum Press: New York, NY, USA, 1987; pp. 399–445. ISBN 978-1-4757-5581-7. [Google Scholar]
- Nan, J.; Spence, J.C.H. On the dose-rate threshold of beam damage in TEM. Ultramicroscopy 2012, 113, 77–82. [Google Scholar]
- Meyer, J.C.; Kotakoski, J.; Mangler, C. Atomic structure from large area, low dose exposure of materials: A new route to circumvent radiation damage. Ultramicroscopy 2014, 145, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Henderson, R. Cryo-protection of protein crystals against radiation damage in electron and X-ray diffractions. Proc. R. Soc. Lond. 1990, B241, 6–8. [Google Scholar]
- Unwin, P.N.T.; Henderson, R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J. Mol. Biol. 1975, 94, 425–440. [Google Scholar] [CrossRef]
- Available online: https://www.nobelprize.org/prizes/chemistry/2017/press-release/ (accessed on 14 February 2020).
- Henderson, R.; Sali, A.; Baker, M.L.; Carragher, B.; Devkota, B.; Downing, K.H.; Egelman, E.H.; Feng, Z.; Frank, J.; Grigorieff, N.; et al. Outcome of the first electron microscopy validation task force meeting. Structure 2012, 20, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühlbrandt, W. The resolution revolution. Science 2014, 343, 1443–1444. [Google Scholar] [CrossRef] [PubMed]
- Faruqi, A.R.; Henderson, R. Electronic detectors for electron microscopy. Curr. Opin. Struct. Biol. 2007, 5, 549–555. [Google Scholar] [CrossRef]
- Merk, A.; Bartesaghi, A.; Banerjee, A.; Falconieri, V.; Rao, P.; Davis, M.I.; Pragani, R.; Boxer, M.B.; Earl, L.A.; Milne, J.L.S.; et al. Breaking Cryo-EM resolution barriers to facilitate drug discovery. Cell 2016, 165, 1698–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egerton, R.F. Mechanisms of radiation damage in beam-sensitive specimens, for TEM accelerating voltages between 10 and 300 kV. Microsc. Res. Tech. 2012, 75, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Egerton, R.F. Choice of operating voltage for a transmission electron microscope. Ultramicroscopy 2014, 145, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Egerton, R.F. Radiation damage to organic and inorganic specimens in the TEM. Micron 2019, 119, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. Television pickup tubes and the problem of vision. In Advances in Electronics; Marton, L., Ed.; Academic Press: New York, NY, USA, 1948; pp. 131–166. [Google Scholar]
- Libera, M.L.; Egerton, R.F. Advances in the transmission electron microscopy of polymers. Polym. Rev. 2010, 50, 321–339. [Google Scholar] [CrossRef]
- Van Dyck, D. Wave reconstruction in TEM using a variable phase plate. Ultramicroscopy 2010, 110, 571–572. [Google Scholar] [CrossRef]
- Gabor, D. A new microscopic principle. Nature 1948, 161, 777–778. [Google Scholar] [CrossRef]
- Lichte, H. Electron holography approaching atomic resolution. Ultramicroscopy 1986, 20, 293–304. [Google Scholar] [CrossRef]
- Lohmann, A. Optische Einseitenbandübertragung angewandt auf das Gabor-Mikroskop. Opt. Acta 1956, 3, 97. [Google Scholar] [CrossRef]
- Leith, E.; Upatnieks, J. Reconstructed wavefronts and communication theory. JOSA 1962, 52, 1123–1128. [Google Scholar] [CrossRef]
- Leith, E.N.; Upatnieks, J. Wavefront reconstruction with continuous-tone objects. JOSA 1963, 53, 1377. [Google Scholar] [CrossRef]
- Testorf, M.; Lohmann, A.W. Holography in phase space. Appl. Opt. 2008, 47, A70–A77. [Google Scholar] [CrossRef]
- Steeds, J.W.; Carlino, E. Electron crystallography. In Electron Microscopy in Materials Science; Merli, P.G., Vittori-Antisari, M., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore; Hackensack, NJ, USA; London, UK; Hong Kong, 1992; pp. 279–313. ISBN 981-02-0924-X. [Google Scholar]
- Lupini, A.R.; Wang, P.; Nellist, P.D.; Kirkland, A.I.; Pennycook, S.J. Aberration measurement using the Ronchigram contrast transfer function. Ultramicroscopy 2010, 110, 891–898. [Google Scholar] [CrossRef]
- Nellist, P.D. Scanning Transmission Electron Microscopy. In Science of Microscopy; Hawkes, P., Spence, J.C.H., Eds.; Springer: New York, NY, USA, 2007; Volume 1, pp. 65–132. ISBN 978-0-387-24296-4. [Google Scholar] [CrossRef]
- Cowley, J.M. Electron diffraction phenomena observed with a high-resolution STEM instrument. Microsc. Res. Tech. 1986, 3, 25–44. [Google Scholar] [CrossRef]
- Goodman, J.W. Introduction to Fourier Optics, 4th ed.; Freeman W. H. and Co. Macmillan Learning: New York, NY, USA, 2017; p. 427, ISBN-13 978-1-319-11916-4. [Google Scholar]
- Latychevskaia, T.; Fink, H.W. Solution to the twin image problem in holography. Phys. Rev. Lett. 2007, 98, 233901–233904. [Google Scholar] [CrossRef] [Green Version]
- Spence, J.C.H.; Carpenter, R.W. Electron microdiffraction. In Principles of Analytical Electron Microscopy; Joy, D.C., Romig, A.D., Jr., Goldstein, J., Eds.; Plenum Press: New York, NY, USA; London, UK, 1986; p. 326. [Google Scholar]
- Ronchi, V. Forty years of history of a grating interferometer. Appl. Opt. 1964, 3, 437–450. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vinpocetine (accessed on 14 February 2020).
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/n-vinyl-2-pyrrolidone (accessed on 14 February 2020).
- Chen, J.Z.A.; Sachse, C.; Xu, C.; Mielke, T.; Spahn, C.M.T.; Grigorieff, N. A dose rate effect in Single-particle electron microscopy. J. Struct. Biol. 2008, 161, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Bloemen, M.; Brullot, W.; Thien Luong, T.; Geukens, N.; Gils, A.; Verbiest, T. Improved functionalization of oleic acid-coated iron oxide nanoparticles for biomedical applications. J. Nanopart. Res. 2012, 14, 1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.-R.; Kisielowski, C.; Van Dyck, D. Prospects for atomic resolution in-line holography for a 3D determination of atomic structures from single projections. Adv. Struct. Chem. Imaging 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischman, S.G.; Kuduva, S.S.; McMahon, J.A.; Moulton, B.; Bailey Walsh, R.D.; Rodriguez-Hornedo, N.; Zaworotko, M.J. Crystal Engineering of the composition of pharmaceutical phases: Multiplecomponent crystalline solids involving Carbamazepine. Cryst. Growth Des. 2003, 3, 909–919. [Google Scholar] [CrossRef]
- Bauer, J.; Spanton, S.; Henry, R.; Quick, J.; Dziki, W.; Porter, W.; Morris, J. Ritonavir: An extraordinary example of conformational polymorphism. Pharm. Res. 2001, 18, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Hasa, D.; Schneider Rauber, G.; Voinovich, D.; Jones, W. Cocrystal formation through mechanochemistry: From neat and liquid-assisted grinding to polymer-assisted grinding. Angew. Chem. Int. Ed. 2015, 54, 7371–7375. [Google Scholar] [CrossRef] [PubMed]
- J E M S-S A A S. Version 4.3931U2016. Available online: http://www.jems-saas.ch/ (accessed on 14 February 2020).
- Banerji, B.; Pramanik, S.K. Binding studies of creatinine and urea on iron nanoparticle. Springerplus 2015, 4, 708. [Google Scholar] [CrossRef] [Green Version]
- Quintana, C.; Cowley, J.M.; Marhic, C. Electron nanodiffraction and high-resolution electron microscopy studies of the structure and composition of physiological and pathological ferritin. J. Struct. Biol. 2004, 147, 166–178. [Google Scholar] [CrossRef]
- Janney, D.E.; Cowley, J.M.; Buseck, P.R. Structure of synthetic 2-line ferrihydrite by electron nanodiffraction. Am. Mineral. 2000, 85, 1180–1187. [Google Scholar] [CrossRef]
- Torres, P.A.; Helmstetter, J.A.; Kaye, M.A.; Kaye, A.D. Rhabdomyolysis: Pathogenesis, diagnosis, and treatment. Ochsner J. 2015, 15, 58–69. [Google Scholar]
- Latychevskaia, T.; Fink, H.W. Practical algorithms for the simulation and reconstruction of digital in-line holograms. Appl. Opt. 2015, 54, 2424–2434. [Google Scholar] [CrossRef] [Green Version]












© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlino, E. In-Line Holography in Transmission Electron Microscopy for the Atomic Resolution Imaging of Single Particle of Radiation-Sensitive Matter. Materials 2020, 13, 1413. https://doi.org/10.3390/ma13061413
Carlino E. In-Line Holography in Transmission Electron Microscopy for the Atomic Resolution Imaging of Single Particle of Radiation-Sensitive Matter. Materials. 2020; 13(6):1413. https://doi.org/10.3390/ma13061413
Chicago/Turabian StyleCarlino, Elvio. 2020. "In-Line Holography in Transmission Electron Microscopy for the Atomic Resolution Imaging of Single Particle of Radiation-Sensitive Matter" Materials 13, no. 6: 1413. https://doi.org/10.3390/ma13061413
APA StyleCarlino, E. (2020). In-Line Holography in Transmission Electron Microscopy for the Atomic Resolution Imaging of Single Particle of Radiation-Sensitive Matter. Materials, 13(6), 1413. https://doi.org/10.3390/ma13061413