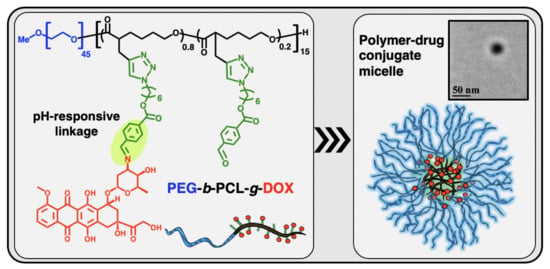
Well-Defined Diblock Poly(ethylene glycol)-b-Poly(ε-caprolactone)-Based Polymer-Drug Conjugate Micelles for pH-Responsive Delivery of Doxorubicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Materials
2.3. Synthesis of Alkyne-Functionalized CL (1)
2.4. Synthesis of Alkyne-Functionalized Poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG-b-PCL, 2)
2.5. Synthesis of 6-Azidohexyl 4-Formylbenzoate (3)
2.6. Synthesis of PEG-b-PCL-g-ALD (4)
2.7. Synthesis of PEG-b-PCL-g-DOX (5)
2.8. DOX Release Study
2.9. Cell Culture
2.10. In Vitro Cytotoxicity Assay
2.11. Cellular Uptake Assay and Confocal Imaging
3. Results and Discussion
3.1. Synthesis
3.2. Characterization
3.3. Drug Release Study
3.4. Cytotoxicity and Cell Internalization Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. C Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Hu, X.; Jing, X. Biodegradable amphiphilic polymer-drug conjugate micelles. Expert Opin. Drug Deliv. 2009, 6, 1079–1090. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Aliabadi, H.M.; Lavasanifar, A. Polymeric micelles for drug delivery. Expert Opin. Drug Deliv. 2006, 3, 139–162. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. Co-delivery of hydrophilic and hydrophobic drugs by micelles: A new approach using drug conjugated PEG–PCLNanoparticles. Drug Dev. Ind. Pharm. 2017, 43, 1908–1918. [Google Scholar] [CrossRef]
- Bae, K.H.; Lee, Y.; Park, T.G. Oil-Encapsulating PEO−PPO−PEO/PEG Shell Cross-Linked Nanocapsules for Target-Specific Delivery of Paclitaxel. Biomacromolecules 2007, 8, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Lavasanifar, A.; Samuel, J.; Kwon, G.S. Poly (ethylene oxide)-block-poly (L-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002, 54, 169–190. [Google Scholar] [CrossRef]
- Lee, J.; Cho, E.C.; Cho, K. Incorporation and release behavior of hydrophobic drug in functionalized poly(d,l-lactide)-block–poly(ethylene oxide) micelles. J. Control. Release 2004, 94, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Jim, T.F.; Li, M.; Yuer, Z.; Wang, S.; Wu, C. Enzymatic Biodegradation of Poly(ethylene oxide-b-ε-caprolactone) Diblock Copolymer and Its Potential Biomedical Applications. Macromolecules 1999, 32, 590–594. [Google Scholar] [CrossRef]
- Mahmud, A.; Xiong, X.-B.; Lavasanifar, A. Novel Self-Associating Poly(ethylene oxide)-block-poly(ε-caprolactone) Block Copolymers with Functional Side Groups on the Polyester Block for Drug Delivery. Macromolecules 2006, 39, 9419–9428. [Google Scholar] [CrossRef]
- Darcos, V.; El Habnouni, S.; Nottelet, B.; El Ghzaoui, A.; Coudane, J. Well-defined PCL-graft-PDMAEMA prepared by ring-opening polymerisation and click chemistry. Polym. Chem. 2010, 1, 280–282. [Google Scholar] [CrossRef]
- Silvers, A.L.; Chang, C.C.; Emrick, T. Functional aliphatic polyesters and nanoparticles prepared by organocatalysis and orthogonal grafting chemistry. J. Polym. Sci. A Polym. Chem. 2012, 50, 3517–3529. [Google Scholar] [CrossRef]
- Silvers, A.L.; Chang, C.-C.; Parrish, B.; Emrick, T. Strategies in Aliphatic Polyester Synthesis for Biomaterial and Drug Delivery Applications. In Degradable Polymers and Materials: Principles and Practice, 2nd ed.; ACS Symposium Series 1114; ACS: Washington, DC, USA, 2012; Chapter 15; pp. 237–254. [Google Scholar]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.A.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T.; et al. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Jafari, A.; Rajabian, N.; Zhang, G.; Alaa Mohamed, M.; Lei, P.; Andreadis, S.T.; Pfeifer, B.A.; Cheng, C. PEGylated Amine-Functionalized Poly(ε-caprolactone) for the Delivery of Plasmid DNA. Materials 2020, 13, 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelegri-O’Day, E.M.; Paluck, S.J.; Maynard, H.D. Substituted Polyesters by Thiol-Ene Modification: Rapid Diversification for Therapeutic Protein Stabilization. J. Am. Chem. Soc. 2017, 139, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, X.J.; Peh, P.; Liao, S.; Sng, C.; Li, J. Controlled drug release from biodegradable thermoresponsive physical hydrogel nanofibers. J. Control. Release 2010, 143, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Pharmacokinetic consequences of pegylation. Drug Deliv. 2006, 13, 399–409. [Google Scholar] [CrossRef]
- Riva, R.; Schmeits, S.; Jérôme, C.; Jérôme, R.; Lecomte, P. Combination of Ring-Opening Polymerization and “Click Chemistry”: Toward Functionalization and Grafting of Poly(ε-caprolactone). Macromolecules 2007, 40, 796–803. [Google Scholar] [CrossRef]
- Parrish, B.; Quansah, J.K.; Emrick, T. Functional polyesters prepared by polymerization of α-allyl(valerolactone) and its copolymerization with ε-caprolactone and δ-valerolactone. J. Polym. Sci. A Polym. Chem. 2002, 40, 1983–1990. [Google Scholar] [CrossRef]
- Iha, R.K.; Wooley, K.L.; Nystrom, A.M.; Burke, D.J.; Kade, M.J.; Hawker, C.J. Applications of orthogonal “click” chemistries in the synthesis of functional soft materials. Chem. Rev. 2009, 109, 5620–5686. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.M.; Xiong, X.-B.; Lu, C.; Lavasanifar, A. Application of Click Chemistry in the Preparation of Poly(ethylene oxide)-block-poly(ε-caprolactone) with Hydrolyzable Cross-Links in the Micellar Core. Macromolecules 2011, 44, 2058–2066. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.-K.; Law, W.-C.; Sun, H.; Prasad, P.N.; Cheng, C. A degradable brush polymer–drug conjugate for pH-responsive release of doxorubicin. Polym. Chem. 2015, 6, 953–961. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.-K.; Law, W.-C.; Weinheimer, E.; Sengupta, S.; Prasad, P.N.; Cheng, C. Polylactide-graft-doxorubicin nanoparticles with precisely controlled drug loading for pH-triggered drug delivery. Biomacromolecules 2014, 15, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Lohmeijer, B.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M. Guanidine and amidine organocatalysts for ring-opening polymerization of cyclic esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Jafari, A.; Sun, H.; Sun, B.; Mohamed, M.A.; Cui, H.; Cheng, C. Layer-by-layer preparation of polyelectrolyte multilayer nanocapsules via crystallized miniemulsions. Chem. Commun. 2019, 55, 1267–1270. [Google Scholar] [CrossRef] [PubMed]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, A.; Yan, L.; Mohamed, M.A.; Wu, Y.; Cheng, C. Well-Defined Diblock Poly(ethylene glycol)-b-Poly(ε-caprolactone)-Based Polymer-Drug Conjugate Micelles for pH-Responsive Delivery of Doxorubicin. Materials 2020, 13, 1510. https://doi.org/10.3390/ma13071510
Jafari A, Yan L, Mohamed MA, Wu Y, Cheng C. Well-Defined Diblock Poly(ethylene glycol)-b-Poly(ε-caprolactone)-Based Polymer-Drug Conjugate Micelles for pH-Responsive Delivery of Doxorubicin. Materials. 2020; 13(7):1510. https://doi.org/10.3390/ma13071510
Chicago/Turabian StyleJafari, Amin, Lingyue Yan, Mohamed Alaa Mohamed, Yun Wu, and Chong Cheng. 2020. "Well-Defined Diblock Poly(ethylene glycol)-b-Poly(ε-caprolactone)-Based Polymer-Drug Conjugate Micelles for pH-Responsive Delivery of Doxorubicin" Materials 13, no. 7: 1510. https://doi.org/10.3390/ma13071510
APA StyleJafari, A., Yan, L., Mohamed, M. A., Wu, Y., & Cheng, C. (2020). Well-Defined Diblock Poly(ethylene glycol)-b-Poly(ε-caprolactone)-Based Polymer-Drug Conjugate Micelles for pH-Responsive Delivery of Doxorubicin. Materials, 13(7), 1510. https://doi.org/10.3390/ma13071510