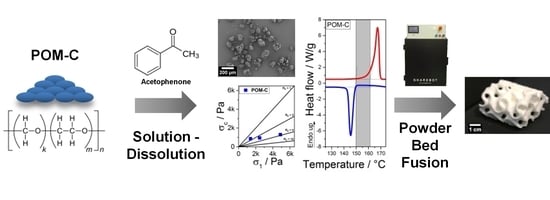
Development of Polyoxymethylene Particles via the Solution-Dissolution Process and Application to the Powder Bed Fusion of Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.2.1. Solution-Dissolution Process
2.2.2. Optical Cloud Point Determination
2.2.3. Powder Bed Fusion of Polymers
2.3. Characterization
2.3.1. Scanning Electron Microscopy
2.3.2. X-ray Diffraction
2.3.3. Laser Diffraction Particle Sizing
2.3.4. Flowability
2.3.5. Nitrogen Sorption Measurements
2.3.6. Differential Scanning Calorimetry
2.3.7. Helium Pycnometry
3. Results
3.1. Solvent Screening
3.2. Manufacturing of POM Feedstock for Powder Bed Fusion
3.2.1. POM-Acetophenone and POM-Triacetin Cloud Points
3.2.2. Effect of POM Concentration on Particle Shape
3.2.3. Thermal Properties and Crystallinity of the Manufactured POM Powders
3.2.4. Product Powder Characterization
3.2.5. Application in the PBF Process
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies, 2nd ed.; Springer: New York, NY, USA, 2015; ISBN 978-1-4939-2113-3. [Google Scholar]
- Brenken, B.; Barocio, E.; Favaloro, A.; Kunc, V.; Pipes, R.B. Fused filament fabrication of fiber-reinforced polymers: A review. Addit. Manuf. 2018, 21, 1–16. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Chatham, C.A.; Long, T.E.; Williams, C.B. A review of the process physics and material screening methods for polymer powder bed fusion additive manufacturing. Prog. Polym. Sci. 2019, 93, 68–95. [Google Scholar] [CrossRef]
- Schmid, M. Laser Sintering with Plastics; Carl Hanser Verlag GmbH & Co. KG: München, Germany, 2018; ISBN 978-1-56990-683-5. [Google Scholar]
- Schmid, M.; Amado, A.; Wegener, K. Polymer powders for selective laser sintering (SLS). In Proceedings of the PPS-30th International Conference of the Polymer Processing Society, Cleveland, OH, USA, 6–12 June 2014; p. 160009. [Google Scholar]
- Wendel, B.; Rietzel, D.; Kühnlein, F.; Feulner, R.; Hülder, G.; Schmachtenberg, E. Additive processing of polymers. Macromol. Mater. Eng. 2008, 293, 799–809. [Google Scholar] [CrossRef]
- Zhao, M.; Wudy, K.; Drummer, D. Crystallization kinetics of polyamide 12 during selective laser sintering. Polymers 2018, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haworth, B.; Hopkinson, N.; Hitt, D.; Zhong, X. Shear viscosity measurements on Polyamide-12 polymers for laser sintering. Rapid Prototyp. J. 2013, 19, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Berretta, S.; Wang, Y.; Davies, R.; Ghita, O.R. Polymer viscosity, particle coalescence and mechanical performance in high-temperature laser sintering. J. Mater. Sci. 2016, 51. [Google Scholar] [CrossRef] [Green Version]
- Berretta, S.; Ghita, O.; Evans, K.E. Morphology of polymeric powders in Laser Sintering (LS): From Polyamide to new PEEK powders. Eur. Polym. J. 2014, 59, 218–229. [Google Scholar] [CrossRef]
- Heinl, M.; Laumer, T.; Bayer, F.; Hausotte, T. Temperature-dependent optical material properties of polymer powders regarding in-situ measurement techniques in additive manufacturing. Polym. Test. 2018, 71, 378–383. [Google Scholar] [CrossRef]
- Wohlers, T.T. Wohlers Report 2016; Wohlers Associates: Fort Collins, CO, USA, 2016; ISBN 978-0-9913332-2-6. [Google Scholar]
- Baumann, F.E.; Wilczok, N. Herstellung von Polyamid—Fällpulvern mit Enger Korngrößenverteilung und Niedriger Porosität. Patent DE 19708946A1, 10 September 1998. [Google Scholar]
- Baumann, F.-E.; Grebe, M.; Monsheimer, S. Laser-Sinter-Pulver mit Einem Metallsalz und Einem Fettsäurederivat, Verfahren zu Dessen Herstellung und Formkörper, Hergestellt aus Diesem Laser-Sinterpulver. Patent DE 10334496A1, 24 February 2005. [Google Scholar]
- Schmidt, J.; Sachs, M.; Fanselow, S.; Zhao, M.; Romeis, S.; Drummer, D.; Wirth, K.E.; Peukert, W. Optimized polybutylene terephthalate powders for selective laser beam melting. Chem. Eng. Sci. 2016, 156, 1–10. [Google Scholar] [CrossRef]
- Sachs, M.; Friedle, M.; Schmidt, J.; Peukert, W.; Wirth, K.-E. Characterization of a downer reactor for particle rounding. Powder Technol. 2017, 316, 357–366. [Google Scholar] [CrossRef]
- Arai, S.; Tsunoda, S.; Kawamura, R.; Kuboyama, K.; Ougizawa, T. Comparison of crystallization characteristics and mechanical properties of poly(butylene terephthalate) processed by laser sintering and injection molding. Mater. Des. 2017, 113, 214–222. [Google Scholar] [CrossRef]
- Gayer, C.; Ritter, J.; Bullemer, M.; Grom, S.; Jauer, L.; Meiners, W.; Pfister, A.; Reinauer, F.; Vučak, M.; Wissenbach, K.; et al. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 101, 660–673. [Google Scholar] [CrossRef]
- Fanselow, S.; Emamjomeh, S.E.; Wirth, K.E.; Schmidt, J.; Peukert, W. Production of spherical wax and polyolefin microparticles by melt emulsification for additive manufacturing. Chem. Eng. Sci. 2016, 141, 282–292. [Google Scholar] [CrossRef]
- Kleijnen, R.; Schmid, M.; Wegener, K. Production and processing of a spherical polybutylene terephthalate powder for laser sintering. Appl. Sci. 2019, 9, 1308. [Google Scholar] [CrossRef] [Green Version]
- Mys, N.; Verberckmoes, A.; Cardon, L. Processing of syndiotactic polystyrene to microspheres for part manufacturing through selective laser sintering. Polymers 2016, 8, 383. [Google Scholar] [CrossRef] [Green Version]
- Kloos, S.; Dechet, M.A.; Peukert, W.; Schmidt, J. Production of spherical semi-crystalline polycarbonate microparticles for Additive Manufacturing by liquid-liquid phase separation. Powder Technol. 2018, 335, 275–284. [Google Scholar] [CrossRef]
- Wang, S.-J.; Liu, J.-Y.; Chu, L.-Q.; Zou, H.; Zhang, S.-J.; Wu, C.-J. Preparation of polypropylene microspheres for selective laser sintering via thermal-induced phase separation: Roles of liquid-liquid phase separation and crystallization. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 320–329. [Google Scholar] [CrossRef]
- Dechet, M.A.; Demina, A.; Römling, L.; Gómez Bonilla, J.S.; Lanyi, F.J.; Schubert, D.W.; Bück, A.; Peukert, W.; Schmidt, J. Development of poly(L-lactide) (PLLA) microspheres precipitated from triacetin for application in powder bed fusion of polymers. Addit. Manuf. 2020, 32, 100966. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Y.; Xu, Y. Preparation of polypropylene powder by dissolution-precipitation method for selective laser sintering. Adv. Polym. Technol. 2019, 2019, 5803895. [Google Scholar] [CrossRef] [Green Version]
- Wegner, A. New polymer materials for the laser sintering process: Polypropylene and others. Phys. Procedia 2016, 83, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Zhang, B.; Song, J.; Bi, G.; Wang, P.; Wei, J. The effect of processing conditions on the mechanical properties of polyethylene produced by selective laser sintering. Polym. Test. 2016, 52, 89–93. [Google Scholar] [CrossRef]
- Strobbe, D.; Dadbakhsh, S.; Verbelen, L.; Van Puyvelde, P.; Kruth, J.-P. Selective laser sintering of polystyrene: A single-layer approach. Plast. Rubber Compos. 2018, 47, 2–8. [Google Scholar] [CrossRef]
- Berretta, S.; Evans, K.E.; Ghita, O. Processability of PEEK, a new polymer for high temperature laser sintering (HT-LS). Eur. Polym. J. 2015, 68, 243–266. [Google Scholar] [CrossRef] [Green Version]
- Ghita, O.; James, E.; Davies, R.; Berretta, S.; Singh, B.; Flint, S.; Evans, K.E. High Temperature Laser Sintering (HT-LS): An investigation into mechanical properties and shrinkage characteristics of Poly (Ether Ketone) (PEK) structures. Mater. Des. 2014, 61, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Chatham, C.A.; Long, T.E.; Williams, C.B. Powder bed fusion of poly(phenylene sulfide)at bed temperatures significantly below melting. Addit. Manuf. 2019, 28, 506–516. [Google Scholar] [CrossRef]
- Dallner, C.; Funkhauser, S.; Müller, F.; Demeter, J.; Völkel, M. Lasersinterpulver aus Polyoxymethylen, Verfahren zu Dessen Herstellung und Formkörper, Hergestellt aus Diesem Lasersinterpulver. Patent WO 2011051250A1, 5 May 2012. [Google Scholar]
- Rietzel, D.; Wendel, B.; Feulner, R.W.; Schmachtenberg, E. New thermoplastic powder for selective laser sintering. Kunstst. Int. 2008, 98, 42–45. [Google Scholar]
- Rietzel, D. Werkstoffverhalten und Prozessanalyse beim Laser-Sintern von Thermoplasten. Ph.D. Thesis, Universität Erlangen-Nürnberg, Erlangen, Germany, 2011. [Google Scholar]
- Van de Witte, P.; Dijkstra, P.J.; Van den Berg, J.W.A.; Feijen, J. Phase separation processes in polymer solutions in relation to membrane formation. J. Memb. Sci. 1996, 117, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, H.; Teramoto, M.; Kuwana, M.; Kitamura, Y. Formation of polypropylene particles via thermally induced phase separation. Polymer 2000, 41, 8673–8679. [Google Scholar] [CrossRef]
- Laxminarayan, A.; McGuire, K.S.; Kim, S.S.; Lloyd, D.R. Effect of initial composition, phase separation temperature and polymer crystallization on the formation of microcellular structures via thermally induced phase separation. Polymer 1994, 35, 3060–3068. [Google Scholar] [CrossRef]
- McGuire, K.S.; Laxminarayan, A.; Lloyd, D.R. Kinetics of droplet growth in liquid-liquid phase separation of polymer-diluent systems: Experimental results. Polymer 1995, 36, 4951–4960. [Google Scholar] [CrossRef]
- Dechet, M.A.; Goblirsch, A.; Romeis, S.; Zhao, M.; Lanyi, F.J.; Kaschta, J.; Schubert, D.W.; Drummer, D.; Peukert, W.; Schmidt, J. Production of polyamide 11 microparticles for Additive Manufacturing by liquid-liquid phase separation and precipitation. Chem. Eng. Sci. 2019, 197, 11–25. [Google Scholar] [CrossRef]
- Schaaf, P.; Lotz, B. Liquid-liquid phase separation and crystallization in binary polymer systems. Polymer 1987, 28, 193–200. [Google Scholar] [CrossRef]
- Aerts, L.; Kunz, M.; Berghmans, H.; Koningsveld, R. Relation between phase behaviour and morphology in polyethylene/diphenyl ether systems. Die Makromol. Chem. 1993, 194, 2697–2712. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Mechadense Gyroid Cylinder. Available online: www.thingiverse.com/thing:41272 (accessed on 23 July 2019).
- 3d-Decoratie Christmas Tree Ornament—Pigeons Flat. Available online: www.thingiverse.com/thing:555902 (accessed on 10 February 2020).
- Schulze, D. Powders Bulk Solids; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-73767-4. [Google Scholar]
- Jenike, A.W. Storage and Flow of Solids (Rev. Ed.); Bulletin of the University of Utah, Volume 53, No. 26; University of Utah: Salt Lake City, UT, USA, 1970. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lorthioir, C.; Lauprêtre, F.; Sharavanan, K.; Lange, R.F.M.; Desbois, P.; Moreau, M.; Vairon, J.P. Solid-state organization and morphological partitioning in polyoxymethylene-based copolymers: A solid-state NMR and WAXS study. Macromolecules 2007, 40, 5001–5013. [Google Scholar] [CrossRef]
- Ehrenstein, G.W.; Riedel, G.; Trawiel, P. Thermal Analysis of Plastics; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2004; ISBN 978-3-446-22673-9. [Google Scholar]
- Runt, J.; Kanchanasopa, M. Crystallinity determination. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Jaffe, M.; Wunderlich, B. Melting of polyoxymethylene. Kolloid Z. Z. Polym. 1967, 216–217, 203–216. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2007; ISBN 978-0-8493-7248-3. [Google Scholar]
- Jabbari, M.; Lundin, M.; Bahadorikhalili, S. Finding solvent for polyamide 11 using a computer software. Zeitschrift für Phys. Chemie 2020, 234, 517–529. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical-and less classical-solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Rehage, G.; Möller, D.; Ernst, O. Entmischungserscheinungen in Lösungen von molekularuneinheitlichen Hochpolymeren. Die Makromol. Chem. 1965, 88, 232–255. [Google Scholar] [CrossRef]
- Van Emmerik, P.T.; Smolders, C.A. Phase separation in polymer solutions. I. Liquid-liquid phase separation of PPO poly (2, 6-dimethyl 1, 4-phenylene oxide) in binary mixtures with toluene and ternary mixtures with toluene and ethyl alcohol. J. Polym. Sci. Part C Polym. Symp. 1972, 38, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Van Emmerik, P.T.; Smolders, C.A. Phase separation in polymer solutions. II. Determination of thermodynamic parameters of poly (2,6-dimethyl-1,4-phenylene oxide) toluene mixtures. J. Polym. Sci. Part C Polym. Symp. 1972, 39, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, H.; Teramoto, M.; Kudari, S.; Kitamura, Y. Effect of diluents on membrane formation via thermally induced phase separation. J. Appl. Polym. Sci. 2001, 82, 169–177. [Google Scholar] [CrossRef]
- Van Emmerik, P.T.; Smolders, C.A. Liquid-liquid phase separation by nucleation and growth in solutions of poly(2,6 dimethyl-1,4 phenylene oxide) in toluene. Eur. Polym. J. 1973, 9, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Lexow, M.M.; Drummer, D. New materials for SLS: The use of antistatic and flow agents. J. Powder Technol. 2016, 2016, 4101089. [Google Scholar] [CrossRef] [Green Version]
- Wudy, K.; Drummer, D. Aging behavior of polyamide 12: Interrelation between bulk characteristics and part properties. In Proceedings of the 26th Annual International Solid Freeform Fabrication Symposium, Austin, TX, USA, 17–19 August 2016; pp. 770–781. [Google Scholar]
- Rumpf, H. Die wissenschaft des agglomerierens. Chem. Ing. Tech. CIT 1974, 46, 1–11. [Google Scholar] [CrossRef]
- Yang, J.; Sliva, A.; Banerjee, A.; Dave, R.N.; Pfeffer, R. Dry particle coating for improving the flowability of cohesive powders. Powder Technol. 2005, 158, 21–33. [Google Scholar] [CrossRef]
- Sutton, A.T.; Kriewall, C.S.; Leu, M.C.; Newkirk, J.W. Powder characterisation techniques and effects of powder characteristics on part properties in powder-bed fusion processes. Virtual Phys. Prototyp. 2017, 12, 3–29. [Google Scholar] [CrossRef]
- Ranellucci, A. Slic3r. 2018. Available online: https://slic3r.org (accessed on 16 October 2018).








| Polymer/Solvent | δd (MPa1/2) | δp (MPa1/2) | δh (MPa1/2) | δt (MPa1/2) | Δδt (MPa1/2) |
|---|---|---|---|---|---|
| POM | 17.1 | 3.1 | 10.7 | 20.41 | — |
| Acetophenone | 18.8 | 9 | 4 | 21.22 | 0.81 |
| Benzaldehyde | 19.4 | 7.4 | 5.3 | 21.43 | 1.02 |
| Benzyl benzoate | 20 | 5-1 | 5.2 | 21.28 | 0.87 |
| Benzyl ether | 19.6 | 3.4 | 5.2 | 20.56 | 0.15 |
| DEGBEA | 16 | 4.1 | 8.2 | 18.44 | 1.97 |
| Triacetin | 16.5 | 4.5 | 9.1 | 19.37 | 1.04 |
| Sample | Tons,m (°C) | Tons,c (°C) | Process Window (K) | Crystallinity | Crystallinity |
|---|---|---|---|---|---|
| Acetophenone | |||||
| 20 wt.% | 163.4 | 149.2 | 14.2 | 83.3 | 88.8 |
| 22 wt.% | 163.4 | 148.8 | 14.6 | 91.9 | 89.2 |
| Triacetin | |||||
| 18 wt.% | 142.6 | 140.0/145.9* | 2.6/—* | 40.4 | 37.0 |
| 20 wt.% | 143.9 | 140.8 | 3.1 | 56.5 | 53.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dechet, M.A.; Baumeister, I.; Schmidt, J. Development of Polyoxymethylene Particles via the Solution-Dissolution Process and Application to the Powder Bed Fusion of Polymers. Materials 2020, 13, 1535. https://doi.org/10.3390/ma13071535
Dechet MA, Baumeister I, Schmidt J. Development of Polyoxymethylene Particles via the Solution-Dissolution Process and Application to the Powder Bed Fusion of Polymers. Materials. 2020; 13(7):1535. https://doi.org/10.3390/ma13071535
Chicago/Turabian StyleDechet, Maximilian A., Ina Baumeister, and Jochen Schmidt. 2020. "Development of Polyoxymethylene Particles via the Solution-Dissolution Process and Application to the Powder Bed Fusion of Polymers" Materials 13, no. 7: 1535. https://doi.org/10.3390/ma13071535
APA StyleDechet, M. A., Baumeister, I., & Schmidt, J. (2020). Development of Polyoxymethylene Particles via the Solution-Dissolution Process and Application to the Powder Bed Fusion of Polymers. Materials, 13(7), 1535. https://doi.org/10.3390/ma13071535