Carvacrol Prodrugs with Antimicrobial Activity Loaded on Clay Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Conversion of Carvacrol Prodrugs
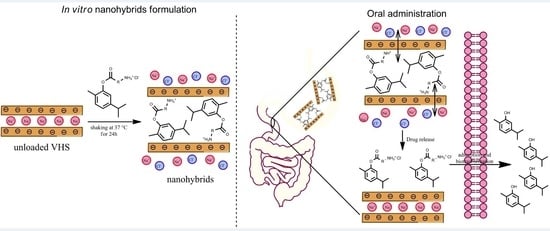
2.2. Preparation of WSCPs/VHS Hybrids
2.3. Solid State Characterization
2.3.1. X-ray Power Diffraction (XRPD)
2.3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.3. Thermal Analysis
2.3.4. In Vitro Release Studies
2.4. Statistical Analysis
3. Results and Discussion
3.1. Chemical Conversion of Carvacrol Prodrugs
3.2. Solid State Characterization of Drug–Clay Hybrids
3.2.1. Adsorption Equilibrium Studies
3.2.2. XRPD
3.3. TGA and DSC
3.4. Fourier Transform Infrared (FTIR)
3.5. In Vitro Release Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 5 March 2020).
- Kumar, A.; Chordia, N. Bacterial Resistance Against Antibiotics. In Drug Resistance in Bacteria, Fungi, Malaria, and Cancer; Arora, Gunjan, Sajid, Andaleeb, Kalia, V.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-48682-6. [Google Scholar]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Antonia, N. Teresa Papalia Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008, 88, 2371–2381. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, J.; Huang, X.; Yu, H.; Xue, F. In vitro evaluation of the activity of microencapsulated carvacrol against Escherichia coli with K88 pili. J. Appl. Microbiol. 2009, 107, 1781–1788. [Google Scholar] [CrossRef]
- de Lange, C.F.M.; Pluske, J.; Gong, J.; Nyachoti, C.M. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Bassanetti, I.; Carcelli, M.; Buschini, A.; Montalbano, S.; Leonardi, G.; Pelagatti, P.; Tosi, G.; Massi, P.; Fiorentini, L.; Rogolino, D. Investigation of antibacterial activity of new classes of essential oils derivatives. Food Control 2017, 73, 606–612. [Google Scholar] [CrossRef]
- Cacciatore, I.; Di Giulio, M.; Fornasari, E.; Di Stefano, A.; Cerasa, L.S.; Marinelli, L.; Turkez, H.; Di Campli, E.; Di Bartolomeo, S.; Robuffo, I.; et al. Carvacrol codrugs: A new approach in the antimicrobial plan. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jornada, D.H.; Dos Santos Fernandes, G.F.; Chiba, D.E.; De Melo, T.R.F.; Dos Santos, J.L.; Chung, M.C. The prodrug approach: A successful tool for improving drug solubility. Molecules 2016, 21, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinelli, L.; Fornasari, E.; Eusepi, P.; Ciulla, M.; Genovese, S.; Epifano, F.; Cacciatore, I. Carvacrol prodrugs as antimicrobial agents. Eur. J. Med. Chem. 2019, 178, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Ene, M.; Biță, B.; Ionescu, C.; Crăciun, L.; Sârbu, I. In Vitro Human Microbiota Response to Exposure to Silver Nanoparticles Biosynthesized with Mushroom. Extract. Nutr. 2018, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity. Nanomaterials 2019, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Maestrelli, F.; Cirri, M.; García-Villén, F.; Borrego-Sánchez, A.; Iborra, C.V.; Mura, P. Tablets of “hydrochlorothiazide in cyclodextrin in nanoclay”: A new nanohybrid system with enhanced dissolution properties. Pharmaceutics 2020, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lu, X.; Su, Y.; Kun, E.; Zhang, F. Clay-Polymer Nanocomposites Prepared by Reactive Melt Extrusion for Sustained Drug Release. Pharmaceutics 2020, 12, 51. [Google Scholar] [CrossRef] [Green Version]
- Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Aguzzi, C.; Viseras, C.; Caramella, C. Clay minerals for tissue regeneration, repair, and engineering. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 385–402. ISBN 9781782424567. [Google Scholar]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Jayrajsinh, S.; Shankar, G.; Agrawal, Y.K.; Bakre, L. Montmorillonite nanoclay as a multifaceted drug-delivery carrier: A review. J. Drug Deliv. Sci. Technol. 2017, 39, 200–209. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, J.H.; Ryu, H.J.; Elzatahry, A.A.; Alothman, Z.A.; Choy, J.H. Drug–clay nanohybrids as sustained delivery systems. Appl. Clay Sci. 2016, 130, 20–32. [Google Scholar] [CrossRef]
- Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Aguzzi, C.; Viseras, C.; Sainz-Díaz, C.I.; Cerezo, P. Assessment of halloysite nanotubes as vehicles of isoniazid. Colloids Surf. B Biointerfaces 2017, 160, 337–344. [Google Scholar] [CrossRef] [PubMed]
- García-Villén, F.; Carazo, E.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; Cerezo, P.; Viseras, C.; Aguzzi, C. Clay minerals in drug delivery systems. In Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-814617-0. [Google Scholar]
- Viseras, C.; Carazo, E.; Borrego-Sánchez, A.; García-Villén, F.; Sánchez-Espejo, R.; Cerezo, P.; Aguzzi, C. Clay minerals in skin drug delivery. Clays Clay Miner. 2019, 67, 59–71. [Google Scholar] [CrossRef]
- Cacciatore, I.; Fornasari, E.; Marinelli, L.; Eusepi, P.; Ciulla, M.; Ozdemir, O.; Tatar, A.; Turkez, H.; Di Stefano, A. Memantine-derived drugs as potential antitumor agents for the treatment of glioblastoma. Eur. J. Pharm. Sci. 2017, 109, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, A.; Liu, B.; Liang, J. Tramadol hydrochloride/montmorillonite composite: Preparation and controlled drug release. Appl. Clay Sci. 2010, 49, 108–112. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Aguzzi, C.; Viseras, C. Hybrid systems based on “ drug – in cyclodextrin – in nanoclays ” for improving oxaprozin dissolution properties. Int. J. Pharm. 2016, 509, 8–15. [Google Scholar] [CrossRef]
- Aguzzi, C.; Viseras, C.; Cerezo, P.; Salcedo, I.; Sánchez-Espejo, R.; Valenzuela, C. Release kinetics of 5-aminosalicylic acid from halloysite. Colloids Surf. B Biointerfaces 2013, 105, 75–80. [Google Scholar] [CrossRef]
- Borrego-Sánchez, A.; Carazo, E.; Aguzzi, C.; Viseras, C.; Sainz-Díaz, C.I. Biopharmaceutical improvement of praziquantel by interaction with montmorillonite and sepiolite. Appl. Clay Sci. 2018, 160, 173–179. [Google Scholar] [CrossRef]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological parameters for oral delivery and in vitro testing. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- Oberle, R.L.; Chen, T.S.; Lloyd, C.; Amidon, G.L.; Barnett, J.L.; Owyang, C.; Meyer, J. The Influence of the Interdigestive Migrating Motor Complex on Gastric-Emptying of Liquids. Gastroenterology 1988, 94, A328. [Google Scholar]
- McConnell, E.L.; Fadda, H.M.; Basit, A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008, 364, 213–226. [Google Scholar] [CrossRef]
- Vig, B.; Rautio, J. Amino acid pro drugs for oral delivery: Challenges and opportunities. Ther. Deliv. 2011, 2, 959–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baertschi, S.W.; Alsante, K.M. Santafianos Dinos Stress testing: The chemistry of drug degradation. In Pharmaceutical Stress Testing: Predicting Drug Degradation; Baertschi, S.W., Alsante, K.M., Reed, R.A., Eds.; Informa Healthcare: London, UK, 2011; pp. 49–141. ISBN 9781616310011. [Google Scholar]
- Yu, W.H.; Li, N.; Tong, D.S.; Zhou, C.H.; Lin, C.X.; Xu, C.Y. Adsorption of proteins and nucleic acids on clay minerals and their interactions: A review. Appl. Clay Sci. 2013, 80–81, 443–452. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Sandri, G.; Ferrari, F.; Rossi, S.; Bonferoni, C.; Caramella, C.; Viseras, C. Intercalation of tetracycline into layered clay mineral material for drug delivery purposes. Mater. Technol. 2014, 29, B96–B99. [Google Scholar] [CrossRef]
- Joshi, G.V.; Kevadiya, B.D.; Patel, H.A.; Bajaj, H.C.; Jasra, R.V. Montmorillonite as a drug delivery system: Intercalation and in vitro release of timolol maleate. Int. J. Pharm. 2009, 374, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Viseras, C.; Cerezo, P.; Rossi, S.; Ferrari, F.; López-Galindo, A.; Caramella, C. Influence of dispersion conditions of two pharmaceutical grade clays on their interaction with some tetracyclines. Appl. Clay Sci. 2005, 30, 79–86. [Google Scholar] [CrossRef]
- Park, J.K.; Choy, Y.B.; Oh, J.M.; Kim, J.Y.; Hwang, S.J.; Choy, J.H. Controlled release of donepezil intercalated in smectite clays. Int. J. Pharm. 2008, 359, 198–204. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Sparks, D.L.; Scrivner, N.C. Sorption and Desorption of Quaternary Amine Cations on Clays. Environ. Sci. Technol. 1993, 27, 1625–1631. [Google Scholar] [CrossRef]
- Lakshmi, M.S.; Sriranjani, M.; Bakrudeen, H.B.; Kannan, A.S.; Mandal, A.B.; Reddy, B.S.R. Carvedilol/montmorillonite: Processing, characterization and release studies. Appl. Clay Sci. 2010, 48, 589–593. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Influence of structure on chemical and thermal stability of aliphatic diesters. J. Phys. Chem. B 2013, 117, 14754–14762. [Google Scholar] [CrossRef]
- Feng, Y.C.; Zhao, H.; Hao, T.H.; Hu, G.H.; Jiang, T.; Zhang, Q.C. Effects of poly(cyclohexanedimethylene terephthalate) on microstructures, crystallization behavior and properties of the poly(ester ether) elastomers. Materials 2017, 10, 694. [Google Scholar] [CrossRef] [Green Version]
- Carazo, E.; Borrego-Sánchez, A.; Sánchez-Espejo, R.; García-Villén, F.; Cerezo, P.; Aguzzi, C.; Viseras, C. Kinetic and thermodynamic assessment on isoniazid/montmorillonite adsorption. Appl. Clay Sci. 2018, 165, 82–90. [Google Scholar] [CrossRef]
- Sivaramakrishna, D.; Swamy, M.J. Structure, supramolecular organization and phase behavior of N-acyl-β-alanines: Structural homologues of mammalian brain constituents N-acylglycine and N-acyl-GABA. Chem. Phys. Lipids 2016, 201, 01–10. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishna, D.; Swamy, M.J. Self-Assembly, Supramolecular Organization, and Phase Behavior of l -Alanine Alkyl Esters (n = 9-18) and Characterization of Equimolar l -Alanine Lauryl Ester/Lauryl Sulfate Catanionic Complex. Langmuir 2015, 31, 9546–9556. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, Y.; Mohamadou, A.; Boudesocque, S.; Hubert, J.; Plantier-Royon, R.; Dupont, L. Ionic liquids derived from esters of Glycine Betaine: Synthesis and characterization. J. Mol. Liq. 2015, 207, 60–66. [Google Scholar] [CrossRef]
- Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of Oregano (Origanum onites L.) Essential Oil in β-Cyclodextrin (β-CD): Synthesis and Characterization of the Inclusion Complexes. Bioengineering 2017, 4, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Preparation and characterization of phosphonium montmorillonite with enhanced thermal stability. Appl. Clay Sci. 2007, 35, 194–200. [Google Scholar] [CrossRef]
- Elkhalifah, A.E.I.; Murugesan, T.; Bustam, M.A. Characterization of different cationic forms of montmorillonite by FTIR, XRD and TGA techniques. In Proceedings of the 2011 IEEE National Postgraduate Conference (NPC), Kuala Lumpur, Malaysia, 19–20 September 2011; pp. 1–6. [Google Scholar]
- Guzel, S.; Unal, H.I.; Ozlem, E.; Sari, B. Polyindene/Organo-Montmorillonite Conducting Nanocomposites. I. Synthesis, Characterization, and Electrokinetic Properties. J. Appl. Polym. Sci. 2012, 123, 2911–2922. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 08, 726–741. [Google Scholar] [CrossRef] [Green Version]
- de Mesquita, B.M.; do Nascimento, P.G.G.; Souza, L.G.S.; de Farias, I.F.; Romézio, A.C. Synthesis, larvicidal and acetylcholinesterase inhibitory activities of carvacrol/thymol and derivatives. Quimica Nova 2018, 41, 412–416. [Google Scholar] [CrossRef]
- Silva, V.B.; Travassos, D.L.; Nepel, A.; Barison, A.; Costa, E.V.; Scotti, L.; Scotti, M.T.; Mendonça-Junior, F.J.B.; dos Santos, R.L.C.; Cavalcanti, S.C.H. Synthesis and chemometrics of thymol and carvacrol derivatives as larvicides against Aedes aegypti. J. Arthropod Borne Dis. 2017, 11, 305–320. [Google Scholar]
- Forteza, M.; Galán, E.; Cornejo, J. Interaction of dexamethasone and montmorillonite—Adsorption-degradation process. Appl. Clay Sci. 1989, 4, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Porubcan, L.S.; Born, G.S.; White, J.L.; Hem, S.L. Interaction of digoxin and montmorillonite: Mechanism of adsorption and degradation. J. Pharm. Sci. 1979, 68, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.V.; Patel, H.A.; Kevadiya, B.D.; Bajaj, H.C. Montmorillonite intercalated with vitamin B1as drug carrier. Appl. Clay Sci. 2009, 45, 248–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.C.; Yu, H.; Zhu, J.; de Lange, K.; Yin, Y.; Wang, Q.; Gong, J. Evaluation of alginate-whey protein microcapsules for intestinal delivery of lipophilic compounds in pigs. J. Sci. Food Agric. 2016, 96, 2674–2681. [Google Scholar] [CrossRef] [PubMed]






| pH | t1/2/Kobs | WSCP1 | WSCP2 | WSCP3 |
|---|---|---|---|---|
| pH 6.8 | t1/2 (h) | 2.33 (±0.07) | 0.76 (±0.01) | 18.17 (±0.10) |
| Kobs (h−1) | 0.2982 (±0.0106) | 0.9027 (±0.0042) | 0.0381 (±0.0002) | |
| pH 7.4 | t1/2 (h) | 4.37 (±0.37) | 0.88 (±0.07) | 16.66 (±0.23) |
| Kobs (h−1) | 0.1937 (±0.0671) | 0.7878 (±0.0629) | 0.0420 (±0.0006) |
| Sample | Temperature (°C) Range of Each Step | DSC Event (°C) | TGA Weight Loss (% w/w) | Thermal Event |
|---|---|---|---|---|
| VHS | 40–120 | 87 (endo) | 6.7 | Desorption of hydration water |
| 600–750 | - | 4.3 | VHS dehydroxylation | |
| WSCP1 | - | 125 | 0.0 | Solid–solid phase transition (polymorphism) |
| - | 166 | 0.0 | Crystal melting point | |
| 170–350 | 224 | ~80.0 | Thermolysis of the ester group, subsequent evaporation of carvacrol moiety | |
| 450–600 | - | ~15.0 | Oxidation of the aminoacidic portion | |
| WSCP2 | - | 165 | 0.0 | Crystal melting point |
| 170–350 | 248 | ~90 | Thermolysis of the ester group, subsequent evaporation of carvacrol moiety | |
| 450–600 | ~10 | Oxidation of the aminoacidic portion | ||
| WSCP-3 | - | 53 | 0.0 | Solid–solid phase transition (polymorphism) |
| - | 81 | 0.0 | Crystal melting point | |
| 170–350 | 241 | ~80.0 | Thermolysis of the ester group, subsequent evaporation of carvacrol moiety | |
| 450–600 | - | ~15.0 | Oxidation of the aminoacidic portion | |
| WSCP1-VHS | 40–100 | 60 | 3.3 | Evaporation of hydration water |
| 170-–350 | - | 13.5 | Drug decomposition | |
| 500–750 | - | 7.2 | Overlapped aminoacidic drug portion oxidation and VHS dehydroxylation | |
| WSCP2-VHS | 40–100 | - | ~50 | Evaporation of hydration water |
| 170–350 | 267 | ~15 | Drug decomposition | |
| 500–750 | - | 5.7 | Overlapped aminoacidic drug portion oxidation and VHS dehydroxylation | |
| WSCP3-VHS | 40–100 | 68 | 2.3 | Evaporation of hydration water |
| 170–350 | - | 16.1 | Drug decomposition | |
| 500–750 | - | 5.2 | Overlapped aminoacidic drug portion oxidation and VHS dehydroxylation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eusepi, P.; Marinelli, L.; García-Villén, F.; Borrego-Sánchez, A.; Cacciatore, I.; Di Stefano, A.; Viseras, C. Carvacrol Prodrugs with Antimicrobial Activity Loaded on Clay Nanocomposites. Materials 2020, 13, 1793. https://doi.org/10.3390/ma13071793
Eusepi P, Marinelli L, García-Villén F, Borrego-Sánchez A, Cacciatore I, Di Stefano A, Viseras C. Carvacrol Prodrugs with Antimicrobial Activity Loaded on Clay Nanocomposites. Materials. 2020; 13(7):1793. https://doi.org/10.3390/ma13071793
Chicago/Turabian StyleEusepi, Piera, Lisa Marinelli, Fátima García-Villén, Ana Borrego-Sánchez, Ivana Cacciatore, Antonio Di Stefano, and Cesar Viseras. 2020. "Carvacrol Prodrugs with Antimicrobial Activity Loaded on Clay Nanocomposites" Materials 13, no. 7: 1793. https://doi.org/10.3390/ma13071793
APA StyleEusepi, P., Marinelli, L., García-Villén, F., Borrego-Sánchez, A., Cacciatore, I., Di Stefano, A., & Viseras, C. (2020). Carvacrol Prodrugs with Antimicrobial Activity Loaded on Clay Nanocomposites. Materials, 13(7), 1793. https://doi.org/10.3390/ma13071793