A Review on Bubble Stability in Fresh Concrete: Mechanisms and Main Factors
Abstract
:1. Introduction
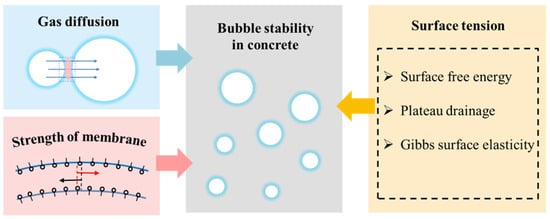
2. Formation and Collapse of Air Bubbles in Concrete
2.1. Formation of Bubbles in Concrete
2.2. Collapse of Bubbles in Concrete
2.2.1. Gas Diffusion
2.2.2. Drainage of Liquid Film
3. Factors Affecting the Stability of Air Bubbles in Fresh Concrete
3.1. Properties of Fresh Concrete
3.1.1. Surface Tension
3.1.2. Viscosity of Fresh Concrete
3.2. Bubble Properties
3.2.1. Surface Charge of Liquid Film
3.2.2. Strength of Liquid Film
3.2.3. Diffusion Rate of Gas through Liquid Film
3.3. Admixtures
3.3.1. Air-Entraining Agent
3.3.2. Superplasticizer
3.3.3. Salt Admixtures
3.4. Mixing Process
3.4.1. Mixing Method
3.4.2. Mixing Time
3.4.3. Mixing Rate
3.5. Transportation, Pumping and Vibration
3.6. Environmental Factors
3.6.1. Temperature
3.6.2. Atmospheric Pressure
4. Measures to Improve the Stability of Air Bubbles in Fresh Concrete
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pigeon, M.; Pleau, R. Durability of Concrete in Cold Climates; E & FN SPON: London, UK, 1996. [Google Scholar]
- Ley, M.T.; Welchel, D.; Peery, J.; Khatibmasjedi, S.; Le Flore, J. Determining the air-void distribution in fresh concrete with the Sequential Air Method. Constr. Build. Mater. 2017, 150, 723–737. [Google Scholar] [CrossRef]
- Xu, G.Q.; Wang, J.C. Analysis of Durability Repair and Protection Technology for Concrete Bridge Piers of Qinghai-Tibet Railway. High-speed Heavy-duty and General Railway Bridge Tunnel Operation Management. Insp. Repair Technol. 2010, 173–176. (In Chinese) [Google Scholar]
- Wang, C.F.; Pei, H.B. Structural problems and durability damage of concrete beams on the Qinghai-Tibet Railway. Railw. Constr. 2004, 8, 8–10. (In Chinese) [Google Scholar]
- Xie, Y.J.; Jia, Y.D.; Zhang, Y. Alkali-aggregate reaction of concrete in construction of Qinghai-Tibet Railway. J. China Railw. Soc. Eng. 2004, 2, 8–15. (In Chinese) [Google Scholar]
- Qin, X.H.; Meng, S.P.; Cao, D.F.; Tu, Y.M.; Sabourova, N.; Grip, N.; Ohlsson, U.; Blanksvärd, T.; Sas, G.; Elfgren, L. Evaluation of freeze-thaw damage on concrete material and prestressed concrete specimens. Constr. Build. Mater. 2016, 125, 892–904. [Google Scholar] [CrossRef]
- Saboori, A. Application of Damage Mechanics to Describe the Behavior of Concrete under Fatigue and Freeze-Thaw Processes. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 2015; pp. 3–22. [Google Scholar]
- Chen, S.P. Freeze-Thaw Damage Model for Different Types of Concrete. Adv. Mater. Res. 2014, 1065–1069, 1776–1779. [Google Scholar] [CrossRef]
- Jan, W.G. Air Entraining Agents; Springer: New York, NY, USA, 2011. [Google Scholar]
- Chatterji, S. Freezing of air-entrained cement-based materials and specific actions of air-entraining agents. Cem. Concr. Compos. 2003, 25, 759–765. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, P.; Zhang, Z.; Pan, Y.; Zhang, W. Effects of Parameters of Air-Avid Structure on the Salt-Frost Durability of Hardened Concrete. Appl. Sci. 2020, 10, 632. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Q.; Li, W.P.; Qin, L.L. Experimental study on concrete performance of superplasticizer and air-entraining agent in Three Gorges Project. Concrete 2000, 7, 20–23. (In Chinese) [Google Scholar]
- Zhang, Y.H. Experimental Research on Concrete Freezing and Thawing of the Second Line of Qinghai-Tibet Railway. New Build. Mater. 2007, 8, 5–8. (In Chinese) [Google Scholar]
- Choi, P.; Yeon, J.H.; Yun, K. Air-void structure, strength, and permeability of wet-mix shotcrete before and after shotcreting operation: The influences of silica fume and air-entraining agent. Cem. Concr. Compos. 2016, 70, 69–77. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.Y.; Geert, D.S.; Ye, G.; Sun, W. Fractal and multifractal analysis on pore structure in cement paste. Constr. Build. Mater. 2014, 69, 253–261. [Google Scholar] [CrossRef]
- Moradian, M.; Hu, Q.; Aboustait, M.; Ley, M.T.; Hanan, J.C.; Xiao, X.H.; Scherer, G.W.; Zhang, Z.D. Direct observation of void evolution during cement hydration. Mater. Des. 2017, 136, 137–149. [Google Scholar] [CrossRef]
- Peter, C.; Hewlett, M.L. Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier Ltd.: Amsterdam, The Netherland, 2019. [Google Scholar]
- Scherer, G.W.; Valenza, J.J. Mechanisms of frost damage. Mater. Sci. Concr. 2005, 7, 209–246. [Google Scholar]
- Jin, S.; Zhang, J.; Huang, B. Fractal analysis of effect of air void on freeze–thaw resistance of concrete. Constr. Build. Mater. 2013, 47, 126–130. [Google Scholar] [CrossRef]
- Powers, T.C.; Helmuth, R.A. Theory of Volume Changes in Hardened Portland-Cement Paste during Freezing. Highw. Res. Board Proc. 1953, 32, 285–297. [Google Scholar]
- Wong, H.S.; Pappas, A.M.; Zimmerman, R.W.; Buenfeld, N.R. Effect of entrained air voids on the microstructure and mass transport properties of concrete. Cem. Concr. Res. 2011, 41, 1067–1077. [Google Scholar] [CrossRef] [Green Version]
- Hover, K.C. Air content and density of hardened concrete. Astm Int. 2006, 296–319. [Google Scholar]
- Neville, A.M. Properties of Concrete, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Hilal, A.A.; Thom, N.H.; Dawson, A.R. On entrained pore size distribution of foamed concrete. Constr. Build. Mater. 2015, 75, 227–233. [Google Scholar] [CrossRef]
- Wee, T.; Babu, D.S.; Tamilselvan, T.; Lim, H. Air-Void System of Foamed Concrete and its Effect on Mechanical Properties. ACI Mater. J. 2006, 103, 45–50. [Google Scholar]
- Nguyen, T.T.; Bui, H.H.; Ngo, T.D.; Nguyen, G.D. Experimental and numerical investigation of influence of air-voids on the compressive behaviour of foamed concrete. Mater. Des. 2017, 130, 103–119. [Google Scholar] [CrossRef]
- Deo, O.; Neithalath, N. Compressive behavior of pervious concretes and a quantification of the influence of random pore structure features. Mater. Sci. Eng. A 2015, 528, 402–412. [Google Scholar] [CrossRef]
- Das, B.B.; Kondraivendhan, B. Implication of pore size distribution parameters on compressive strength, permeability and hydraulic diffusivity of concrete. Constr. Build. Mater. 2012, 28, 382–386. [Google Scholar] [CrossRef]
- Wawrzeńczyk, J.; Kozak, W. A Method of Analyzing the Porous Microstructure in Air-Entrained Concrete on the Basis on 2D Image Analysis. Procedia Eng. 2015, 108, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Mayercsik, N.P.; Vandamme, M.; Kurtis, K.E. Assessing the efficiency of entrained air voids for freeze-thaw durability through modeling. Cem. Concr. Res. 2016, 88, 43–59. [Google Scholar] [CrossRef]
- Powers, T.C. The requirement of frost resistance concrete. Proc. Highw. Res. Board. 1949, 29, 194–211. [Google Scholar]
- Koenig, A. Analysis of air voids in cementitious materials using micro X-ray computed tomography (µXCT). Constr. Build. Mater. 2020, 244, 118313–118326. [Google Scholar] [CrossRef]
- Zhao, Z.H. Analysis of factors affecting concrete gas content. Organizing Committee of “Building Technology and Management”. In Proceedings of the April 2018 Building Technology and Management Academic Exchange Conference. Committee: Beijing Hengsheng Boya International Cultural Exchange Center, Beijing, China, 9–10 July 2018. (In Chinese). [Google Scholar]
- Wang, C.M. Analysis of influencing factors of concrete gas content and control measures. Port Eng. Technol. 2015, 52, 77–79. (In Chinese) [Google Scholar]
- Wang, W.Z.; Feng, Z.X. Influencing factors of air content in fresh concrete. J. Chang. Univ. 2009, 29, 107–110. (In Chinese) [Google Scholar]
- Zhu, B.B.; Wu, X.L.; Huang, S.L. Influencing factors of formation and stability of air bubble system in concrete. Concrete 1999, 2, 13–16. (In Chinese) [Google Scholar]
- Zhang, Y.Q. Several factors affecting the gas content of concrete mixtures. Concr. Cem. Prod. 1998, 2, 5–8. (In Chinese) [Google Scholar]
- Fu, C.H. Study on the Effect of Mixing Method on the Air Content and Performance of Concrete. Master’s Thesis, Chang’an University, Xi’an, China, 2011. (In Chinese). [Google Scholar]
- Jiang, P.; Jiang, L.; Zha, J.; Song, Z. Influence of temperature history on chloride diffusion in high volume fly ash concrete. Constr. Build. Mater. 2017, 144, 677–685. [Google Scholar] [CrossRef]
- Li, X.F.; Fu, Z. Influence of low pressure environment on concrete gas content and bubble stability. J. Chin. Ceram. Soc. 2015, 43, 1076–1082. (In Chinese) [Google Scholar]
- Corr, D.J.; Juenger, M.C.G.; Monteiro, P.J.M.; Bastacky, J. Investigating Entrained Air Voids and Portland Cement Hydration with Low-Temperature Scanning Electron Microscopy. Cem. Concr. Compos. 2004, 26, 1007–1012. [Google Scholar] [CrossRef]
- Zhou, W.L.; Shang, Y. Influence of vibrating method and air-entraining agent type on concrete bubble structure. Ind. Constr. 2009, 39, 958–960. (In Chinese) [Google Scholar]
- Gagné, R. 17-Air entraining agents. Sci. Technol. Concr. Admix. 2016, 379–391. [Google Scholar]
- Zhang, Z. Synthesis of Polycarboxylic Acid Water Reducer and Its Air Entrainment and Early Strength Properties. Master’s Thesis, South China University of Technology, Gunagzhou, China, 2010. (In Chinese). [Google Scholar]
- Ruckenstein, E.; Bhakta, A. Effect of Surfactant and Salt Concentrations on the Drainage and Collapse of Foams Involving Ionic Surfactants. Langmuir 1996, 12, 4134–4144. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Spörel, F.; Uebachs, S.; Brameshuber, W. Investigations on the influence of fly ash on the formation and stability of artificially entrained air voids in concrete. Mater. Struct. 2009, 42, 227–240. [Google Scholar] [CrossRef]
- Pigeon, M.; Plante, P.; Plante, M. Air-void stability. Part I: Influence of silica fume and other parameters. Mater. J. 1989, 86, 482–490. [Google Scholar]
- Haustein, E.; Kuryłowicz-Cudowska, A. The Effect of Fly Ash Microspheres on the Pore Structure of Concrete. Minerals 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.; Emarah, D.A. Resistance of concrete containing ternary cementitious blends to chloride attack and carbonation. J. Mater. Res. Technol. 2020, 1, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Zheng, T. Analysis of influencing factors of fluctuations in gas content of hydraulic concrete. Sichuan Hydropower 2014, 33, 62–65. (In Chinese) [Google Scholar]
- Li, X.C.; Deng, D.H.; He, F.Q. Study on factors influencing air content of air-entrained concrete. Low Temp. Build. Technol. 2008, 1, 17–19. (In Chinese) [Google Scholar]
- Zhao, X.J. Discussion on the influencing factors of concrete gas content. China Sci. Technol. Inf. 2005, 2, 143–150. (In Chinese) [Google Scholar]
- Wei, E.L.; Ma, J.F. Factors affecting the air content of concrete. Shandong Build. Mater. 2004, 6, 61–62. (In Chinese) [Google Scholar]
- Lv, L.H.; Liu, J.Z.; Li, Y.S. Study on influencing factors of air content in concrete. Low Temp. Build. Technol. 2004, 6, 3–4. (In Chinese) [Google Scholar]
- Fantous, T.; Yahia, A. Air-void characteristics in highly flowable cement-based materials. Constr. Build. Mater. 2020, 235, 117454–117466. [Google Scholar] [CrossRef]
- Hulshizer, A.J. Air-entrainment control or consequences. Concr. Int. 1997, 18, 38–40. [Google Scholar]
- Mielenz, R.C.; Wolkodoff, V.E.; Backstrom, J.S. Origin, evolution, and effects of the air void system in concrete: Part 1: Entrained air in unhardened concrete. ACI J. Proc. 1958, 55, 5–122. [Google Scholar]
- Bhattacharya., S.; Hebert., D.; Kresta., S.M. Air entrainment in baffled stirred tanks. Chem. Eng. Res. Des 2007, 85, 654–664. [Google Scholar] [CrossRef]
- Princen, H.M.; Mason, S.G. The permeability of soap films to gases. J. Colloid Interface Sci. 1967, 20, 353–375. [Google Scholar] [CrossRef]
- Caskey, J.A.; Barlage, W.B. An improved experimental technique for determining dynamic surface tension of water and surfactant solutions. J. Colloid Interface Sci. 1971, 35, 46–52. [Google Scholar] [CrossRef]
- Powers, T.C. The Properties of Fresh Concrete; John Wiley & Sons: New York, NY, USA, 1968. [Google Scholar]
- Powers, T.C. Void spacing as a basic for producing air-entrained concrete. Am. Concr. Inst. 1954, 25, 741–760. [Google Scholar]
- Davies, J.T. An Introduction to Eddy Transfer of Momentum, Mass and Heat, Particularly at Interfaces, Turbulence Phenomena; Academic Press: New York, NY, USA, 1972. [Google Scholar]
- Cazacliu, B.; Legrand, J. Characterization of the granular-to-fluid state process during mixing by power evolution in a planetary concrete mixer. Chem. Eng. Sci. 2008, 63, 4617–4630. [Google Scholar] [CrossRef]
- Chopin, D.; Cazacliu, B.; Larrard, F.; Schell, R. Monitoring of concrete homogenization with the power consumption curve. Mater. Struct. 2007, 40, 897–907. [Google Scholar] [CrossRef]
- Zeng, X.H. Study on Material Characteristics of Cement Emulsified Asphalt Mortar and Quality Control of Filling Layer Construction. Ph.D. Thesis, Central South University, Changsha, China, 2010. (In Chinese). [Google Scholar]
- Wang, X.C. A Study of Surface Properties and Foam Properties of Model Surfactant Systems for Foam Combination Flooding. Ph.D. Thesis, Graduate University of Chinese Academy of Sciences, Beijing, China, 2008. (In Chinese). [Google Scholar]
- Chu, P.; Finch, J.; Bournival, G.; Ata, S.; Hamlett, C.; Pugh, R.J. A review of bubble break-up. Adv. Colloid Interface Sci. 2019, 270, 108–122. [Google Scholar] [CrossRef]
- Tse, K.L.; Martin, T.; McFarlane, C.M.; Nienow, A.W. Small bubble formation via a coalescence dependent break-up mechanism. Chem. Eng. Sci. 2003, 58, 275–286. [Google Scholar] [CrossRef]
- Myers, D. Surfaces, Interfaces, and Colloids: Principles and Applications, 2nd ed.; Wiley-VCH: New York, NY, USA, 1999. [Google Scholar]
- Rosen. Surfactants and Interfacial Phenomena, 4th ed.; Colloids & Surfaces: Amsterdam, The Netherland, 2012. [Google Scholar]
- Ley, M.T.; Chancey, R.; Juenger, M.C.G.; Folliard, K.J. The physical and chemical characteristics of the shell of Air-Entrained Bubbles in Cement Paste. Cem. Concr. Res. 2009, 39, 417–425. [Google Scholar] [CrossRef]
- Yang, S.D. Study on the Influence of Separation Pressure and Surface Viscosity on Liquid Membrane Drainage Process. Ph.D. Thesis, North China Electric Power University, Beijing, China, 2018. (In Chinese). [Google Scholar]
- Zhang, C.R. Study on the Interface Expansion Viscoelasticity and Foam Properties of Foam Composite Flooding Simulation System. Ph.D. Thesis, Graduate School of Chinese Academy of Sciences, Beijing, China, 2007. (In Chinese). [Google Scholar]
- Slastanova, A.; Campbell, R.A.; Snow, T.; Mould, E.; Li, P.X.; Welbourn, R.J.L.; Chen, M.; Robles, E.; Briscoe, W.H. Synergy, competition, and the “hanging” polymer layer: Interactions between a neutral amphiphilic ‘tardigrade’ comb co-polymer with an anionic surfactant at the air-water interface. J. Colloid Interface Sci. 2020, 561, 181–194. [Google Scholar] [CrossRef]
- Borkowski, M.; Kosior, D.; Zawala, J. Effect of initial adsorption coverage and dynamic adsorption layer formation at bubble surface in stability of single foam films. Colloid Surf. A Phys. Eng. Asp. 2020, 589, 1–25. [Google Scholar] [CrossRef]
- Botta, O.D.; Magos, I.; Balan, C. Experimental study on the formation and break-up of fluid bubbles. Incas Bull. 2020, 12, 27–34. [Google Scholar] [CrossRef]
- Boubendir, L.; Chikh, S.; Tadrist, L. On the surface tension role in bubble growth and detachment in a micro-tube. Int. J. Multiph. Flow. 2020, 124, 103196. [Google Scholar] [CrossRef]
- Söderlund, T.; Alakoskela, J.I.; Pakkanen, A.L.; Kinnunen, P.K.J. Comparison of the Effects of Surface Tension and Osmotic Pressure on the Interfacial Hydration of a Fluid Phospholipid Bilayer. Biophys. J. 2003, 85, 2333–2341. [Google Scholar] [CrossRef] [Green Version]
- Behzadian., R.; Shahrajabian., H. Experimental Study of the Effect of Nano-silica on the Mechanical Properties of Concrete/PET Composites. KSCE J. Civ. Eng. 2019, 23, 3660–3668. [Google Scholar] [CrossRef]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid Interface Sci. 2015, 222, 228–259. [Google Scholar] [CrossRef]
- Stubenrauch, C.; Miller, R. Stability of Foam Films and Surface Rheology: An Oscillating Bubble Study at Low Frequencies. J. Phys. Chem. B 2004, 108, 6412–6421. [Google Scholar] [CrossRef]
- Krause, J.P. Foams: Theory, Measurements, and Applications; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 57–65. [Google Scholar]
- Szecsy, R.S. Concrete Rheology. Ph.D. Dissertation, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 1997. [Google Scholar]
- Rath, S.; Ouchi, M.; Puthipad, N.; Attachaiyawuth, A. Improving the stability of entrained air in self-compacting concrete by optimizing the mix viscosity and air entraining agent dosage. Constr. Build. Mater. 2017, 148, 531–537. [Google Scholar] [CrossRef]
- Han, D.; Lee, G.C.; Yoon, S.J.; Kim, K.M. Viscosity influence on rising behavior of model air bubbles in fresh mortar. Constr. Build. Mater. 2015, 76, 10–15. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Ltd.: London, UK, 1997. [Google Scholar]
- Huang, D. Structural and Rheological Properties of Fresh Concrete; China Construction Industry Press: Beijing, China, 1983. (In Chinese) [Google Scholar]
- Mazanec, O.; Lowke, D.; Schiessl, P. Mixing of high performance concrete: Effect of concrete composition and mixing intensity on mixing time. Mater. Struct. 2009, 43, 357–365. [Google Scholar] [CrossRef]
- Fei, X.J. Hydraulics of Slurry and Granular Material Transportation; Tsinghua University Press: Beijing, China, 1994. (In Chinese) [Google Scholar]
- Łaźniewska-Piekarczyk, B. Examining the possibility to estimate the influence of admixtures on pore structure of self-compacting concrete using the air void analyzer. Constr. Build. Mater. 2013, 41, 374–387. [Google Scholar] [CrossRef]
- Du, L.; Folliard, K.J. Mechanisms of air entrainment in concrete. Cem. Concr. Res. 2005, 35, 1463–1471. [Google Scholar] [CrossRef]
- Wang., J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Colloid Interface Sci. 2015, 228, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, A.; Ruckenstein, E. Decay of standing foams: Drainage, coalescence and collapse. Adv. Colloid Interface Sci. 1997, 70, 1–124. [Google Scholar] [CrossRef]
- Tabakova, S.S.; Danov, K.D. Effect of disjoining pressure on the drainage and relaxation dynamics of liquid films with mobile interfaces. J. Colloid Interface Sci. 2009, 2336, 273–284. [Google Scholar] [CrossRef]
- Carey, E.; Stubenrauch, C. A disjoining pressure study of foam films stabilized by mixtures of a nonionic (C12DMPO) and an ionic surfactant (C12TAB). J. Colloid Interface Sci. 2010, 343, 314–323. [Google Scholar] [CrossRef]
- Ding, B.; Liu, J.P.; Liu, J.Z. Study on the Stabilization Mechanism of Air-entraining Agents and Improving the Freeze-Thaw Durability of Concrete. Concrete 2006, 11, 34–35. (In Chinese) [Google Scholar]
- Yuan, X.F. Study on the Preparation and Performance of Frost-Resistant Concrete. Master’s Thesis, Chongqing University, Chongqing, China, 2016. (In Chinese). [Google Scholar]
- Matar, O.K. Nonlinear evolution of thin free viscous films in the presence of soluble surfactant. Phys. Fluids 2002, 14, 4216–4234. [Google Scholar] [CrossRef]
- Boussinesq, J. Velocity spherical drop specific gravity. Ann. Chim. Phys. 1913, 29, 349–354. [Google Scholar]
- Li, Y.; Jia, S.Y. Effect of Surface Viscosity on Coalescence of Small Bubbles. J. Chem. Ind. Eng. 1995, 5, 532–538. (In Chinese) [Google Scholar]
- Lopez, J.M.; Hirsa, A.H. Surfactant-Influenced Gas-Liquid Interfaces: Nonlinear Equation of State and Finite Surface Viscosities. J. Colloid Interface Sci. 2000, 229, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naire, S.; Braun, R.J.; Snow, S.A. An Insoluble Surfactant Model for a Vertical Draining Free Film. J. Colloid Interface Sci. 2000, 230, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Naire, S.; Braun, R.J.; Snow, S.A. A 2+1dimensional insoluble surfactant model for a vertical draining free film. J. Comput. Appl. Math. 2002, 166, 385–410. [Google Scholar] [CrossRef] [Green Version]
- Naire, S.; Braun, R.J.; Snow, S.A. An insoluble surfactant model for a vertical draining free film with variable surface viscosity. Phys. Fluids 2001, 13, 2492–2502. [Google Scholar] [CrossRef]
- Heidari, A.H.; Braun, R.J.; Hirsa, A.H.; Snow, S.A.; Naire, S. Hydrodynamics of a Bounded Vertical Film with Nonlinear Surface Properties. J. Colloid Interface Sci. 2002, 253, 295–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saulnier, L.; Champougny, L.; Bastien, G.; Restagno, F.; Langevin, D.; Rio, E. A study of generation and rupture of soap films. Soft Matter. 2014, 10, 2899–2906. [Google Scholar] [CrossRef]
- Sett, S.; Sinha-Ray, S.; Yarin, A.L. Gravitational drainage of foam films. J. Colloid Interface Sci. 2013, 29, 4934–4947. [Google Scholar] [CrossRef]
- Zang, D.Y.; Rio, E.; Delon, G.; Langevin, D.; Wei, B.; Binks, B.P. Influence of the contact angle of silica nanoparticles at the air–water interface on the mechanical properties of the layers composed of these particles. Mol. Phys. 2011, 109, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Chang, C.L.; Yang, W.C.; Zhang, B.S.; Yuan, J.; Yu, J.S. Effects of common inorganic salts on solution surface tension and concrete properties. Concrete 2007, 6, 7–9. (In Chinese) [Google Scholar]
- Diao, S. Study on High Temperature and High Salt Foam System and Its Performance. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2006. (In Chinese). [Google Scholar]
- Tunstall, L.E.; Scherer, G.W.; Prud’homme, R.K. Studying AEA interaction in cement systems using tensiometry. Cem. Concr. Res. 2017, 92, 29–36. [Google Scholar] [CrossRef]
- Pham, L.T.; Cramer, S.M. Comparison of Fresh Concrete Air Content Test Methods & Analysis of Hardened Air Content in Wisconsin Pavements. Ph.D. Thesis, University of Wisconsin–Madison, Madison, WI, USA, 2019. [Google Scholar]
- Mezhov, A.; Ulka, S.; Gendel, Y.; Diesendruck, C.E.; Kovler, K. The working mechanisms of low molecular weight polynaphthalene sulfonate superplasticizers. Constr. Build. Mater. 2020, 240, 117891–117899. [Google Scholar] [CrossRef]
- Plante, P.; Pigeon, M.; Saucier, F. Air-void stability, Part II: Influence of superplasticizers and cement. Mater. J. 1989, 86, 581–589. [Google Scholar]
- Macinnis, C.; Racic, D. The effect of superplasticizers on the entrained air-void system in concrete. Cem. Concr. Res. 1986, 16, 345–352. [Google Scholar] [CrossRef]
- Szwabowski, J.; Lazniewska-Piekarczyk, B. Air-entrainment problem in self-compacting concrete. J. Civ. Eng. Manag. 2009, 15, 137–147. [Google Scholar] [CrossRef]
- Liu, J.P.; Shang, Y.; Miao, C.W. Effect of polycarboxylic acid superplasticizer air-entrainment method on concrete performance. J. Build. Mater. 2011, 14, 528–531. (In Chinese) [Google Scholar]
- Ma, J.F.; Shang, Y.Z.; Peng, C.G.; Liu, H.L.; Zheng, S.Z.; Zhao, H.X.; Qi, S.; Ran, Q.P. Synthesis, characterization, and performance of novel phosphate-modified polymers as air-entraining agents. Constr. Build. Mater. 2020, 232, 117231–117240. [Google Scholar] [CrossRef]
- Li, Q.F. Effects of Salt Admixtures on Pore Structure of Air-Entrained Concrete. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2010. (In Chinese). [Google Scholar]
- Carey, C.S. Foaming Properties of Mixtures of a Non-Ionic(C12DMPO) and an Ionic Surfactant (C12TAB). J. Colloid Interface Sci. 2010, 346, 414–423. [Google Scholar] [CrossRef]
- Dean, S.W.; Zhang, S.; Wang, K. Investigation into the effects of materials and mixing procedures on air void characteristics of fresh concrete using air void analyzer (AVA). J. ASTM Int. 2006, 3, 1246–1251. [Google Scholar] [CrossRef]
- Deng, A.M. Commercial Concrete Machinery; People’s Communications Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wang, B. Mechanism and Experimental Study of Improving Concrete Performance by Vibration Mixing. Master’s Thesis, Chang’an University, Xian, China, 2014. (In Chinese). [Google Scholar]
- Fu, C.H. Study on the Influence of Mixing Method on the Air Content and Performance of Cement Concrete. Ph.D. Thesis, Chang’an University, Xian, China, 2011. (In Chinese). [Google Scholar]
- Fu, C.H.; Feng, Z.X.; Zhang, L. Effect of Mixing Method on Air Content and Pore Structure of Concrete. J. Zhengzhou Univ. 2011, 32, 42–45. (In Chinese) [Google Scholar]
- Feng, Z.X. Research Progress of Stirring Theory and Equipment. Eng. Mach. 2014, 45, 1–8. (In Chinese) [Google Scholar]
- Charonnat, Y.; Beitzel, H. efficiency of concrete mixers; report: Efficiency of concrete mixers towards qualification of mixer. Mater. Struct. 1997, 30, 28–32. [Google Scholar] [CrossRef]
- Schiessl, P.; Mazanec, O.; Lowke, D. SCC and UHPC—Effect of Mixing Technology on Fresh Concrete Properties; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Ran, Y.Z. Study on Cement-based Materials Stirring Dispersion and Air Entrainment Characteristics in Low Pressure Environment. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2019. (In Chinese). [Google Scholar]
- Jia, D. Control of Bubble Introduction in Ultra-High Performance Concrete Mixing and Its Effect on Strength. Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2017. (In Chinese). [Google Scholar]
- Wang, W.Z.; Feng, Z.X. Analysis of the Effect of Mixing Speed on the Air Content of Freshly Mixed Concrete. Concrete 2007, 8, 83–88. (In Chinese) [Google Scholar]
- Lessard, M.; Baalbaki, M.; Aitcin, P.C. Effect of pumping on air characteristics of conventional concrete. Trans. Res. Rec. North 1996, 1532, 1–8. [Google Scholar] [CrossRef]
- Hover, K.C.; Phares, R.J. Impact of concrete placing method on air content, air-void system parameters, and freeze–thaw durability. Trans. Res. Rec. North 1996, 1532, 9–14. [Google Scholar] [CrossRef]
- Hover, K.C. Vibration tune-up: Monitoring vibrator frequency to optimize concrete strength, density, and durability. Concr. Int. 2001, 32, 31–35. [Google Scholar]
- Li, Z.J.; Li, Q.; Miao, A.Y. Test on guarantee measures of frost resistance of pumped concrete for long-distance transportation. Water Transp. Eng. 2009, 12, 113–115. (In Chinese) [Google Scholar]
- Liu, J.H.; Yu, D.Y.; Li, Z.H. Time-dependent change of gas content in offshore high-performance concrete. J. Ocean Univ. China 2016, 46, 104–109. (In Chinese) [Google Scholar]
- Zhang, Y.C.; Gao, L.L. Effect of High Frequency Vibration on Pore Parameters and Frost Resistance of Air Entrained Concrete. Adv. Mater. Res. 2013, 671-674, 1680–1683. [Google Scholar] [CrossRef]
- Dodson, V.H. Concrete Admixtures; Van Nostrand Reinhold: New York, NY, USA, 1990. [Google Scholar]
- Tattersall, G.H. Relationships between the British Standard Tests for Workability and The Two-Point Test. Mag. Concr. Res. 1976, 28, 143–147. [Google Scholar] [CrossRef]
- Huo, J.Y.; Wang, Z.J.; Chen, H.X.; He, R. Impacts of Low Atmospheric Pressure on Properties of Cement Concrete in Plateau Areas: A Literature Review. Materials 2019, 12, 1384. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yang, H.Q.; Zhou, S.H.; Wang, A.; Lv, X.D. Effect of Atmospheric Pressure on Performance of AEA and Air Entraining Concrete. Adv. Mater. Sci. Eng. 2018, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.H.; Xie, Y.J.; Zhang, Y. Influence of ambient air pressure on concrete gas content. Concrete 2004, 4, 9–10. [Google Scholar]
- Zhu, C.H. Experimental Research on High Performance Concrete in Permafrost Regions of the Qinghai-Tibet Plateau. Master’s Thesis, Academy of Railway Science, Leshan, China, 2004. (In Chinese). [Google Scholar]
- Wang, Y.J.; Zhang, L.C.; Luo, S.Z. The effect of lower atmospheric pressure on the surface tension coefficient of water bodies. Sci. Technol. Eng. 2017, 17, 136–138. (In Chinese) [Google Scholar]
- Luo, Z.; Fu, Z.; LI, X. Effect of atmospheric pressure on air content and air void parameters of concrete. Mag. Concr. Res. 2015, 67, 1–10. [Google Scholar]
- Li, X.F.; Fu, Z. Influence of plateau low pressure environment on air content and air bubble stability of air-entrained concrete. J. Agric. Eng. 2015, 31, 165–172. (In Chinese) [Google Scholar]
- Li, X.F.; Fu, Z.; Luo, Y. Study on the Influence of Low Pressure Environment on the Air Content and Stomatal Structure of Concrete. Highw. Commun. Sci. Technol. 2015, 32, 49–54. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.F.; Fu, Z.; Luo, Y. The influence of low-pressure environment on plateau on the gas content of fresh concrete. J. Southeast Univ. 2014, 44, 1046–1051. (In Chinese) [Google Scholar]
- Li, X.F. Research on the Anti-Freezing Design and Preventive Measures of Concrete in Qinghai-Tibet Plateau. Ph.D. Thesis, Southeast University, Nanjing, China, 2015. (In Chinese). [Google Scholar]
- Chen, Z.Z. Research on the Anti-Freezing Design and Measures of Concrete in Qinghai-Tibet Plateau. Ph.D. Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2017. (In Chinese). [Google Scholar]
- Li, X.F. The influence of air pressure environment on the performance of different air-entraining agents and the pore structure of air-entraining concrete. Trans. Chin. Soc. Agric. Eng. 2018, 34, 144–150. (In Chinese) [Google Scholar]
- Ke, G.J.; Tian, B.; Li, X.F. Investigation and Analysis of Cement Concrete of Structures on the South Route of the Sichuan-Tibet Highway (Tibet Border). Ind. Constr. Mag. 2015, 8, 58–65. (In Chinese) [Google Scholar]
- Li, Y.; Wang, Z.D.; Wang, L. Effect of Low Air Pressure on Air Bubble Generation and Development of Air-Entrainer Solution. Concrete 2019, 8, 144–148. (In Chinese) [Google Scholar]
- Wang, L.J.; Zhang, G.Y.; Dong, J.F. Advances in Testing and Evaluation Methods of Foam Properties. Dly. Chem. Ind. 2005, 3, 171–173. (In Chinese) [Google Scholar]
- Zamanian, M.; Kolahchi, R.; Bidgoli, M.R. Agglomeration effects on the buckling behaviour of embedded concrete columns reinforced with SiO2 nano-particles. Wind Struct. 2017, 24, 43–57. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Foam flow in a layered, heterogeneous porous medium: A visualization study. Fuel 2017, 197, 58–69. [Google Scholar] [CrossRef]
- Ardalan, R.B.; Jamshidi, N.; Arabameri, H.; Joshaghani, A.; Mehrinejad, M.; Sharafi, P. Enhancing the permeability and abrasion resistance of concrete using colloidal nano-Si oxide and spraying nanosilicon practices. Constr. Build. Mater. 2017, 146, 128–135. [Google Scholar] [CrossRef]
- MENDES, T.M.; REPETTE, W.L. Effect of nano-silica on Portland cement matrix. Rev. Ibracon Estrut. E Mater. 2019, 12, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Heidari, A.; Tavakoli, D. A study of the mechanical properties of ground ceramic powder concrete incorporating nano-silica particles. Constr. Build. Mater. 2013, 38, 255–259. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.M.; Li, S.Y. Research on oil displacement performance of SiO2 nanoparticle stabilized foam system. J. China Univ. Pet. 2014, 4, 124–131. (In Chinese) [Google Scholar]
- Sun, Q.; Li, Z.M.; Li, S.Y. Surface properties and profile control performance of foam with nano-SiO2 particles added. J. China Univ. Pet. 2016, 40, 101–108. (In Chinese) [Google Scholar]
- Yang, Z.Z.; Zhu, J.Y.; Li, X.G. Research progress of nanoparticle stabilized foam in oil and gas production. Prog. Chem. Ind. 2017, 36, 1675–1681. (In Chinese) [Google Scholar]
- Li, Z.M.; Wang, P.; Li, S.Y. Research progress of nano particles to improve the stability of carbon dioxide foam. J. Southwest Pet. Univ. 2014, 36, 155–161. (In Chinese) [Google Scholar]
- Li, Z.M.; Sun, Q.; Li, S.Y. Study on the mechanism of nanoparticle to improve foam stability. Oilfield Chem. 2013, 30, 625–629. (In Chinese) [Google Scholar]
- Li, Z.M.; Wang, P.; Li, S.Y. Co-stabilizing effect of SiO2 nanoparticles and SDS on CO2 foam. J. Northeast Pet. Univ. 2014, 38, 110–115. (In Chinese) [Google Scholar]
- Lu, Q.C.; Li, Z.M.; Li, B.F. Experimental study on filtration loss of foam system stabilized by nanoparticles and viscoelastic surfactants. China Sci. 2017, 12, 241–248. (In Chinese) [Google Scholar]
- She, W.; Du, Y.; Miao, C.W.; Liu, J.P.; Zhao, G.T.; Jiang, J.Y.; Zhang, Y.S. Application of organic- and nanoparticle-modified foams in foamed concrete: Reinforcement and stabilization mechanisms. Cem. Concr. Res. 2018, 106, 12–22. [Google Scholar] [CrossRef]
- Yekeen, N.; Manan, M.A.; Idris, A.K.; Samin, A.M.; Risal, A.R. Mechanistic study of nanoparticles-surfactant foam flow in etched glass micro-models. J. Dispers. Sci. Technol. 2017, 25, 347–357. [Google Scholar] [CrossRef]
- Petit, P.; Javierre, I.; Jézéquel, P.H.; Biance, A.L. Generation and stability of bubbles in a cement based slurry. Cem. Concr. Res. 2014, 60, 37–44. [Google Scholar] [CrossRef]
- Du, Y. Preparation of Nano-Modified Foamed Concrete and Its Stabilization and Strengthening Mechanism. Master’s Thesis, Southeast University, Nanjing, China, 2018. (In Chinese). [Google Scholar]
- Simatupang, P.H.; Andreas, A.; Nugraha, A. The long-term effects of nano-silica on concrete. Mater. Sci. Eng. 2019, 508, 012038–012043. [Google Scholar]
- Mukharjee, B.B.; Barai, S.V. Influence of Nano-Silica on the properties of recycled aggregate concrete. Constr. Build. Mater. 2014, 55, 29–37. [Google Scholar] [CrossRef]









| Research | Experiment Apparatus/METHOD | Primary Conclusions | Summary |
|---|---|---|---|
| Ding et al. [98] | KSV Langmuir mini-trough | The monolayer strength of the air-entraining agent at gas–liquid interface determined the bubble stability directly. The higher the membrane strength, the more stable the air bubbles entrained by AEA, and the greater durability of concrete. | 1. Surface viscosity and elasticity of bubble film greatly influence the stability of air bubbles. 2. Higher surface viscosity and elasticity could inhibit the liquid drainage of bubble film, which promotes the strength of bubble film. |
| Wang et al. [94] | A literature review | Although different scales of foam structure like the gas-water interface and the liquid film have been explored to clarify the mechanisms that control the foam stability, many questions remain unanswered yet. | |
| Yang [74] | Numerical simulation | The surface viscosity is an important factor affecting the process of liquid film drainage. When the surface viscosity is not considered, the surface of the liquid film presents a “flow” mode; when it is considered, it presents a “rigid” mode. With the increase of the surface viscosity, the drainage rate slows down obviously. | |
| Zhang [75] | Interfacial relaxation of tension experiment | The surface viscoelastic properties of nonionic surfactants NP-8, NP-10 and NP-12 were studied. The system with higher surface elasticity has higher bubble stability. The increase of the surface viscosity of the liquid film inhibits the thinning of the surface film. | |
| Naire et al. [104] | A mathematical model; experiment | The evolution of a vertically oriented thin liquid film drainage under gravity was studied. The results showed that increasing surface viscosity and the Marangoni effect could retard drainage, and consequently enhance film stability. | |
| Naire et al. [106] | A mathematical model | It was verified that in the limit of large surface viscosity and the Marangoni effect, the evolution of the free surface is that of a rigid film. Stable aqueous films can be formed in the regime of high surfactant concentrations. | |
| Saulnier et al. [108] | Film rupture experiments. | The results showed that for surfactants with high surface elastic modulus, the rupture began by the expansion of a thinning zone at the top of the film. The lifetime of films with small surface elastic modulus was much shorter than the ones with rigid interfaces. |
| Research | Testing Apparatus | Primary Conclusions | Summary |
|---|---|---|---|
| Zang et al. [110] | Langmuir trough; Angle Microscope; Rheometer; | Foams prepared with nano-particles possessing intermediate hydrophobicity (i.e., the largest adsorption energy) were the most stable. | SiO2 nano-particles had great effects on bubble stability: 1. promoted the strength of liquid film; 2. increased surface viscosity and elasticity; 3. retarded gas diffusion and liquid drainage of bubble film; 4. prevent coalescence of bubbles. |
| Sun et al. [162,163] | Tracker Interfacial Rheometer | SiO2 nano-particles promoted mechanical strength of the liquid film, reduced the drainage, disproportionation and collapse rate of the bubble; the stability and surface dilatational viscoelasticity of foam increased with the increase of mass fraction of SiO2 nano-particles. | |
| Yang et al. [164] | A literature review | Nano-particles (E.g. Nano silica) adsorbed at interface could increase the surface elasticity of bubble; block the flow of liquid in the film; delay the thinning of film, and prevent the coalescence of bubble. In addition, the foam produced when contact angle is around 90° was stable. | |
| Li et al. [165,166,167] | Tracker interfacial rheometer; viscometer; centrifuge; microscope | Nano-particles could attenuate the drainage of the liquid membrane and reduce the coalescence of bubbles, which played a critical role in protecting bubbles. | |
| Lu et al. [168] | Interface rheometer, microscope; viscosity meter; gas diffusion testing device | The SiO2 nano-particles foam showed excellent resistance to liquid drainage and bubble coalescence. Besides, the strength of the bubble liquid film is relatively high. | |
| She et al. [169] | Viscometer XCT Precision system (YXLON, Germany) | Nano-silica could slow the coalescence and disproportionation of bubbles and increase the viscosity of the bubble wall, thus preventing gas transfer and drainage between gaseous and liquid phases. | |
| Yekeen et al. [170] | Leica EZ4 HD stereo microscope | The presence of SiO2 nano-particles in the surfactant solution improved the foam dynamic stability in water-wet and oil-wet porous media. | |
| Petit et al. [171] | SEM; A device to fabricate stable and fully covered solid cement bubble | Bubble stability is shown to be governed mainly by particle covering rate, which is maximized when the particle wetting angle prior to liquid approaches π/2. | |
| Du [172] | SEM, viscometer Optical microscope | Nano silica had great effects on bubble stability. It enhanced the viscosity of the solution and the strength of film; blocked Plateau channels and nodes, Moreover, bubble size was refined. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Lan, X.; Zhu, H.; Liu, H.; Umar, H.A.; Xie, Y.; Long, G.; Ma, C. A Review on Bubble Stability in Fresh Concrete: Mechanisms and Main Factors. Materials 2020, 13, 1820. https://doi.org/10.3390/ma13081820
Zeng X, Lan X, Zhu H, Liu H, Umar HA, Xie Y, Long G, Ma C. A Review on Bubble Stability in Fresh Concrete: Mechanisms and Main Factors. Materials. 2020; 13(8):1820. https://doi.org/10.3390/ma13081820
Chicago/Turabian StyleZeng, Xiaohui, Xuli Lan, Huasheng Zhu, Haichuan Liu, Hussaini Abdullahi Umar, Youjun Xie, Guangcheng Long, and Cong Ma. 2020. "A Review on Bubble Stability in Fresh Concrete: Mechanisms and Main Factors" Materials 13, no. 8: 1820. https://doi.org/10.3390/ma13081820
APA StyleZeng, X., Lan, X., Zhu, H., Liu, H., Umar, H. A., Xie, Y., Long, G., & Ma, C. (2020). A Review on Bubble Stability in Fresh Concrete: Mechanisms and Main Factors. Materials, 13(8), 1820. https://doi.org/10.3390/ma13081820