Antibacterial PEGylated Solid Lipid Microparticles for Cosmeceutical Purpose: Formulation, Characterization, and Efficacy Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Microspheres’ Melting Temperature Range
2.3. Microspheres Characterization
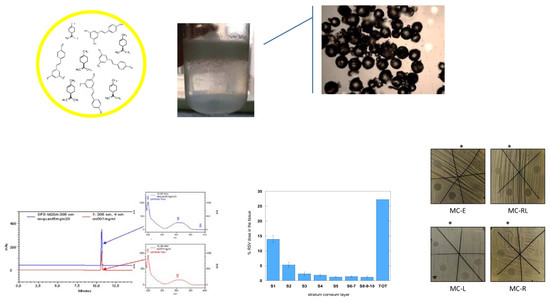
2.3.1. Morphology Analysis
2.3.2. Microspheres’ Size Distribution Analysis
2.3.3. RSV Encapsulation Efficacy (EE) and Drug Loading (DL) Assay
2.3.4. Limonene Detection by Gas Chromatography–Flame Ionization Detector (GC–FID) Analysis
2.3.5. Differential Scanning Calorimetry (DSC) Analysis
2.3.6. Electron Paramagnetic Resonance (EPR) Spectroscopy Analysis
2.3.7. RSV Detection by High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Photostability Evaluation of RSV Entrapped into the Microspheres
2.5. Ex-Vivo Permeation Assay throughout Porcine Ear Skin
2.5.1. Preliminary RSV Solubility Tests
2.5.2. Evaluation of the Tissue Permeation by Vertical Franz Cell Apparatus
2.5.3. Evaluation of Tissue Penetration by Tape-Stripping Assay
2.6. Antibacterial Activity Evaluation of Microspheres
2.7. Data Analysis
3. Results and Discussion
3.1. Screening of Lipids Excipient
3.2. Preparation of RSV and LIM Solid Lipid Microspheres
3.3. Microspheres Characterization
3.3.1. Morphology Analysis and Particle Size Distribution
3.3.2. Drugs Loading Determination
3.3.3. DSC, HPLC, and EPR Analyses Evaluation
3.4. Stability Evaluation of RSV Microencapsulate to the Light Exposure
3.5. Ex-Vivo Permeation Assays
3.5.1. Evaluation of RSV Solubility in Aqueous Solution
3.5.2. Franz Cell Assay
3.5.3. Tape-Stripping Assay
3.6. Antibacterial Activity against Staphylococcus Aureus ATCC 25923
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaeger, B.; Wagemans, F.M.A.; Evans, A.M.; van Beest, I. Effects of facial skin smoothness and blemishes on trait impressions. Perception 2018, 47, 608–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.U.; Schlosser, B.J.; Paller, A.S. A review of diagnosis and treatment of acne in adult female patients. Int. J. Women’s Dermatol. 2017, 4, 56–71. [Google Scholar] [CrossRef]
- Stulberg, D.L.; Penrod, M.A.; Blatny, R.A. Common bacterial skin infections. Am. Fam. Physician 2002, 66, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is caretta caretta a carrier of antibiotic resistance in the mediterranean sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, M.; Gaglio, S.; Galluzzo, P.; Cascone, G.; Piraino, C.; Di Marco Lo Presti, V.; Alduina, R. Antibiotic resistance profiling, analysis of virulence aspects and molecular genotyping of staphylococcus aureus isolated in Sicily, Italy. Foodborne Pathog. Dis. 2018, 15, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Maggio, B.; Raffa, D.; Raimondi, M.V.; Cascioferro, S.; Plescia, F.; Schillaci, D.; Cusimano, M.G.; Leonchiks, A.; Zhulenkovs, D.; Basile, L.; et al. Discovery of a new class of sortase a transpeptidase inhibitors to tackle gram-positive pathogens: 2-(2-phenylhydrazinylidene)alkanoic acids and related derivatives. Molecules 2016, 21, 241. [Google Scholar] [CrossRef] [Green Version]
- Cascioferro, S.; Maggio, B.; Raffa, D.; Raimondi, M.V.; Cusimano, M.G.; Schillaci, D.; Manachini, B.; Plescia, F.; Daidone, G. Synthesis and biofilm formation reduction of pyrazole-4-carboxamide derivatives in some Staphylococcus aureus strains. Eur. J. Med. Chem. 2016, 123, 58–68. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.; García-Conesa, M.; Espín, J. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [Green Version]
- Thapa, S.B.; Pandey, R.P.; Park, Y.I.; Kyung Sohng, J. Biotechnological advances in resveratrol production and its chemical diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [Green Version]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Docherty, J.J.; McEwen, H.A.; Sweet, T.J.; Bailey, E.; Booth, T.D. Resveratrol inhibition of Propionibacterium acnes. J. Antimicrob. Chemother. 2007, 59, 1182–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Murgia, D.; Angellotti, G.; D’Agostino, F.; De Caro, V. Bioadhesive matrix tablets loaded with lipophilic nanoparticles as vehicles for drugs for periodontitis treatment: Development and characterization. Polymers 2019, 11, 1801. [Google Scholar] [CrossRef] [Green Version]
- Erasto, P.; Viljoen, A.M. Limonene—A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.B.; Mehta, A.A. In vitro evaluation of antioxidant activity of D-Limonene. Asian J. Pharm. Pharmacol. 2018, 4, 883–887. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Yan, J.; Sun, Z. D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol. Med. Rep. 2017, 15, 2339–2346. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-H.; Cai, M.; Liu, Y.-S.; Sun, P.-L.; Luo, S.-L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef] [Green Version]
- Nøhr-Meldgaard, K.; Ovsepian, A.; Ingmer, H.; Vestergaard, M. Resveratrol enhances the efficacy of aminoglycosides against Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 390–396. [Google Scholar] [CrossRef]
- Augustin, M.A.; Sanguansri, L.; Lockett, T. Nano-and micro-encapsulated systems for enhancing the delivery of resveratrol. Ann. N. Y. Acad. Sci. 2013, 1290, 107–112. [Google Scholar] [CrossRef]
- Hahn, T.; Hansen, S.; Neumann, D.; Kostka, K.-H.; Lehr, C.-M.; Muys, L.; Schaefer, U.F. Infrared densitometry: A fast and non-destructive method for exact stratum corneum depth calculation for in vitro tape-stripping. Skin Pharmacol. Physiol. 2010, 23, 183–192. [Google Scholar] [CrossRef]
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive polymeric films to enhance barbaloin penetration into buccal mucosa: A novel approach to chemoprevention. AAPS PharmSciTech 2019, 20, 18. [Google Scholar] [CrossRef]
- Del Consuelo, I.D.; Pizzolato, G.P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef]
- De Caro, V.; Murgia, D.; Seidita, F.; Bologna, E.; Alotta, G.; Zingales, M.; Campisi, G. Enhanced in situ availability of aphanizomenon flos-aquae constituents entrapped in buccal films for the treatment of oxidative stress-related oral diseases: Biomechanical characterization and in vitro/ex vivo evaluation. Pharmaceutics 2019, 11, 35. [Google Scholar] [CrossRef] [Green Version]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of skin absorption of drugs from topical and transdermal formulations. Braz. J. Pharm. Sci. 2016, 52. [Google Scholar] [CrossRef] [Green Version]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J.W. The tape stripping procedure—Evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; Zito, P.; Alduina, R.; Ramarosandratana, A.V.; Bruno, M.; Rosselli, S.; Sajeva, M.; Notarbartolo, M. Essential oil composition of alluaudia procera and in vitro biological activity on two drug-resistant models. Molecules 2019, 24, 2871. [Google Scholar] [CrossRef] [Green Version]
- Rubino, S.; Pibiri, I.; Minacori, C.; Alduina, R.; Di Stefano, V.; Orecchio, S.; Buscemi, S.; Girasolo, M.A.; Tesoriere, L.; Attanzio, A. Synthesis, structural characterization, anti-proliferative and antimicrobial activity of binuclear and mononuclear Pt(II) complexes with perfluoroalkyl-heterocyclic ligands. Inorganica Chim. Acta 2018, 483, 180–190. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.; Laveriano-Santos, E.; Marhuenda-Muñoz, M.; Storniolo, C.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R. Health effects of resveratrol: Results from human intervention trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [Green Version]
- Baxter, R.A. Anti-aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef]
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a methoxylated resveratrol derivative, efficiently eradicates planktonic, biofilm, and intracellular mrsa by topical application. Front. Microbiol. 2017, 8, 1103. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-S.; Baik, J.S.; Oh, T.-H.; Yoon, W.-J.; Lee, N.H.; Hyun, C.-G. Biological Activities of Korean citrus obovoides and citrus natsudaidai essential oils against acne-inducing bacteria. Biosci. Biotechnol. Biochem. 2008, 72, 2507–2513. [Google Scholar] [CrossRef] [Green Version]
- Sakeena, M.H.F.; Elrashid, S.M.; Muthanna, F.A.; Ghassan, Z.A.; Kanakal, M.M.; Lia, L.; Munavvar, A.S.; Azmin, M.N. Effect of limonene on permeation enhancement of ketoprofen in palm oil esters nanoemulsion. J. Oleo Sci. 2010, 59, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Sapra, B.; Jain, S.; Tiwary, A.K. Percutaneous permeation enhancement by terpenes: Mechanistic view. AAPS J. 2008, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Jaspart, S.; Piel, G.; Delattre, L.; Evrard, B. Solid lipid microparticles: Formulation, preparation, characterisation, drug release and applications. Expert Opin. Drug Deliv. 2005, 2, 75–87. [Google Scholar] [CrossRef]
- Rabasco Alvarez, A.M.; González Rodríguez, M.L. Lipids in pharmaceutical and cosmetic preparations. Grasas Y Aceites 2000, 51, 74–96. [Google Scholar] [CrossRef] [Green Version]
- Giannola, L.I.; De Caro, V.; Di Stefano, V.; Rizzo, M.C. In vitro evaluation of cumulative release of valproic acid and vitamin E from hexadecanol microspheres. Part 2: Antiepileptic agents. Pharmazie 1993, 48, 917–920. [Google Scholar]
- Togashi, N.; Shiraishi, A.; Nishizaka, M.; Matsuoka, K.; Endo, K.; Hamashima, H.; Inoue, Y. Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 2007, 12, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.G.; Monteiro, J.; Marques, R.R.N.; Silva, A.M.T.; Martínez, C.; Canle, L.M.; Faria, J.L. Photochemical and photocatalytic degradation of trans-resveratrol. Photochem. Photobiol. Sci. 2013, 12, 638–644. [Google Scholar] [CrossRef]
- Kirilov, P.; Tran, V.H.; Ducrotté-Tassel, A.; Salvi, J.-P.; Perrot, S.; Haftek, M.; Boulieu, R.; Pirot, F. Ex-Vivo percutaneous absorption of enrofloxacin: Comparison of LMOG organogel vs. pentravan cream. Int. J. Pharm. 2016, 498, 170–177. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Meyer, W.; Kacza, J.; Zschemisch, N.H.; Godynicki, S.; Seeger, J. Observations on the actual structural conditions in the stratum superficiale dermidis of porcine ear skin, with special reference to its use as model for human skin. Ann. Anat. 2007, 189, 143–156. [Google Scholar] [CrossRef]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Ski. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Mock, C.; Liang, D. Pre-formulation studies of resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef]








| Excipient | RSV (5% w/w) | RSV (10% w/w) | RSV (15% w/w) |
|---|---|---|---|
| Gelucire 62/05 | Opalescent | Opalescent | Opalescent |
| Glyceryl Monostearate | Opalescent | Opalescent | Opalescent |
| Isostearyl Isostearate | Opalescent | Opalescent | Opalescent |
| Labrafil M-1944 | Opalescent | Opalescent | Opalescent |
| Labrafil M-2130 | Opalescent | Opalescent | Opalescent |
| Labrasol | Clear | Clear | Slightly Opalescent |
| Soy Lecithin | Opalescent | Opalescent | Opalescent |
| Tefose 1500 | Clear | Slightly Opalescent | Opalescent |
| Sample | EXA (g) | Labrasol (g) | RSV (g) | LIM (μL) | Mixture M.t.r. (°C) | Microspheres M.t.r. (°C) |
|---|---|---|---|---|---|---|
| MC-RL1 | 0.57 | 0.38 | 0.05 | 10 | 45–51 | 44–47 |
| MC-RL2 | 0.555 | 0.37 | 0.075 | 10 | 47–53 | 43–49 |
| MC-RL3 | 0.462 | 0.462 | 0.075 | 10 | 43–49 | 39–44 |
| MC-R3 | 0.462 | 0.462 | 0.075 | − | 43–49 | 39–44 |
| MC-L3 | 0.500 | 0.500 | − | 10 | 38–42 | 36–40 |
| MC-E | 0.500 | 0.500 | − | − | 38–42 | 36–40 |
| Aqueous Solvents | Solubility (mg/mL) |
|---|---|
| PBS + α-CD 0.5% | 0.153 ± 0.008 |
| PBS + BSA 5% | 0.442 ± 0.035 |
| PBS + β-CD 2% | 0.886 ± 0.011 |
| PBS + DMSO 10% | 0.194 ± 0.010 |
| Citrate buffer (pH 6.2) + β-CD 2% | 0.803 ± 0.003 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angellotti, G.; Murgia, D.; Presentato, A.; D’Oca, M.C.; Scarpaci, A.G.; Alduina, R.; Raimondi, M.V.; De Caro, V. Antibacterial PEGylated Solid Lipid Microparticles for Cosmeceutical Purpose: Formulation, Characterization, and Efficacy Evaluation. Materials 2020, 13, 2073. https://doi.org/10.3390/ma13092073
Angellotti G, Murgia D, Presentato A, D’Oca MC, Scarpaci AG, Alduina R, Raimondi MV, De Caro V. Antibacterial PEGylated Solid Lipid Microparticles for Cosmeceutical Purpose: Formulation, Characterization, and Efficacy Evaluation. Materials. 2020; 13(9):2073. https://doi.org/10.3390/ma13092073
Chicago/Turabian StyleAngellotti, Giuseppe, Denise Murgia, Alessandro Presentato, Maria Cristina D’Oca, Amalia Giulia Scarpaci, Rosa Alduina, Maria Valeria Raimondi, and Viviana De Caro. 2020. "Antibacterial PEGylated Solid Lipid Microparticles for Cosmeceutical Purpose: Formulation, Characterization, and Efficacy Evaluation" Materials 13, no. 9: 2073. https://doi.org/10.3390/ma13092073
APA StyleAngellotti, G., Murgia, D., Presentato, A., D’Oca, M. C., Scarpaci, A. G., Alduina, R., Raimondi, M. V., & De Caro, V. (2020). Antibacterial PEGylated Solid Lipid Microparticles for Cosmeceutical Purpose: Formulation, Characterization, and Efficacy Evaluation. Materials, 13(9), 2073. https://doi.org/10.3390/ma13092073