Evaluation of Cocoa Beans Shell Powder as a Bioadsorbent of Congo Red Dye Aqueous Solutions
Abstract
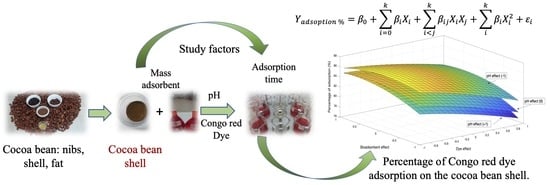
:1. Introduction
2. Materials and Methods
2.1. Conditioning of the Bioadsorbent Material
2.2. Adsorption Studies
2.3. Assessment of the Adsorption Capacity
2.4. Characterization of the Bioadsorbent
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Characterization of the Cocoa Bean Shell Bioadsorbent
3.1.1. Scanning Electron Microscopy (SEM)
3.1.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. Adsorption of Congo Red Dye by the Bioadsorbent from Cocoa Bean Shells
3.3. Effect of pH
3.4. Effect of the Bioadsorbent Concentration
3.5. Effects of the Initial Dye Concentration and the Contact Time
3.6. Optimization of the Adsorption Process
3.7. Comparison with Other Dye Adsorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| Bioadsorbent | Mass of the bioadsorbent (g) |
| ci | Initial concentration (mg L−1) |
| cf | Final concentration (mg L−1) |
| CH2 | Methylene functional group |
| CH | Carbon–hydrogen bond |
| C=O | Carbonyl functional group |
| dm | Dry matter |
| Dye | Concentration (mg L−1) |
| FT IR | Fourier-transform infrared spectroscopy |
| NH2 | Amino functional group |
| OH | Hydroxyl functional group |
| pH | pH value (3–11) |
| RSM | Response surface methodology |
| R2 | Coefficient of determination |
| R2(adjusted) | Adjusted coefficient of determination |
| SEM | Scanning electron microscopy |
| S=O | Sulfate functional group |
| Time | Time (h) |
| X1 | Adimensional linear factor for dye |
| X2 | Adimensional linear factor for bioadsorbent |
| X3 | Adimensional linear factor for pH |
| X4 | Adimensional linear factor for time |
| % A | Adsorption percentage of Congo red dye (%) |
| YAdsorption % | Response variable of adsorption percentage (%) |
| Greek symbols | |
| β | Regression coefficient |
| ε | Random error |
| Subindex | |
| i,j | Index notation of the factors or coefficient |
References
- Bansal, S.; Pandey, P.K.; Upadhayay, S. Methylene Blue Dye Removal from Wastewater Using Ailanthus Excelsa Roxb as Adsorbent. Water Conserv. Sci. Eng. 2021, 6, 1–9. [Google Scholar] [CrossRef]
- Kaçakgil, E.C.; Bingöl, D. Performance assessment and statistical modeling of modification and adsorptive properties of a lignocellulosic waste modified using reagent assisted mechanochemical process as a low-cost and high-performance method. Sustain. Chem. Pharm. 2020, 15, 100226. [Google Scholar] [CrossRef]
- Kadhom, M.; Albayati, N.; Alalwan, H.; Al-Furaiji, M. Removal of dyes by agricultural waste. Sustain. Chem. Pharm. 2020, 16, 100259. [Google Scholar] [CrossRef]
- Da Silva, B.C.; Zanutto, A.; Pietrobelli, J.M. Biosorption of reactive yellow dye by malt bagasse. Adsorpt. Sci. Technol. 2019, 37, 236–259. [Google Scholar] [CrossRef]
- Raghunath, S.; Anand, K.; Gengan, R.; Nayunigari, M.K.; Maity, A. Sorption isotherms, kinetic and optimization process of amino acid proline based polymer nanocomposite for the removal of selected textile dyes from industrial wastewater. J. Photochem. Photobiol. B Biol. 2016, 165, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.; Lima, E.; Pinto, L. Biosorption of food dyes onto Spirulina platensis nanoparticles: Equilibrium isotherm and thermodynamic analysis. Bioresour. Technol. 2012, 103, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, O.S.; Auta, M.; Ayodele, O.B. Ackee apple (Blighia sapida) seeds: A novel adsorbent for the removal of Congo Red dye from aqueous solutions. Chem. Ecol. 2013, 29, 58–71. [Google Scholar] [CrossRef]
- Güler, M.; Çetintaş, S.; Bingöl, D. Cinnamon bark as low-cost and eco-friendly adsorbent for the removal of indigo carmine and malachite green dyestuffs. Int. J. Environ. Anal. Chem. 2021, 101, 735–757. [Google Scholar] [CrossRef]
- Prola, L.D.; Acayanka, E.; Lima, E.C.; Umpierres, C.S.; Vaghetti, J.C.; Santos, W.O.; Laminsi, S.; Djifon, P.T. Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of Reactive Red 120 textile dye from aqueous solution. Ind. Crops Prod. 2013, 46, 328–340. [Google Scholar] [CrossRef]
- Yang, C.-X.; Lei, L.; Zhou, P.-X.; Zhang, Z.; Lei, Z.-Q. Preparation and characterization of poly(AA co PVP)/PGS composite and its application for methylene blue adsorption. J. Colloid Interface Sci. 2015, 443, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xiao, T.; Zhang, J.; Chen, Y.; Li, L. Activated carbon fiber as heterogeneous catalyst of peroxymonosulfate activation for efficient degradation of Acid Orange 7 in aqueous solution. Sep. Purif. Technol. 2015, 143, 19–26. [Google Scholar] [CrossRef]
- Ribeiro, C.; Scheufele, F.; Espinoza-Quiñones, F.R.; Módenes, A.N.; da Silva, M.G.C.; Vieira, M.G.A.; Borba, C.E. Characterization of Oreochromis niloticus fish scales and assessment of their potential on the adsorption of reactive blue 5G dye. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 693–701. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Pinto, I.S.; Amavisca, C.V.; Royer, B.; Pinto, R.B.; Alencar, W.S.; Pereira, S. Application of cupuassu shell as biosorbent for the removal of textile dyes from aqueous solution. J. Environ. Manag. 2011, 92, 1237–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, J.A.; Villanueva, M.E.; Piehl, L.L.; Copello, G.J. Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: Adsorption and desorption study. Chem. Eng. J. 2015, 280, 41–48. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Souza, R.P.; Melo, S.R.; Almeida, V.C. Percolation as new method of preparation of modified biosorbents for pollutants removal. Chem. Eng. J. 2016, 283, 1305–1314. [Google Scholar] [CrossRef]
- Abbas, M.; Trari, M. Kinetic, equilibrium and thermodynamic study on the removal of Congo Red from aqueous solutions by adsorption onto apricot stone. Process. Saf. Environ. Prot. 2015, 98, 424–436. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Mane, V.S.; Babu, P.V.V. Journal of the Taiwan Institute of Chemical Engineers Kinetic and equilibrium studies on the removal of Congo red from aqueous solution using Eucalyptus wood (Eucalyptus globulus) saw dust. J. Taiwan Inst. Chem. Eng. 2013, 44, 81–88. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, A.; Siddiqi, Z.-M. Removal of congo red dye from aqueous system using Phoenix dactylifera seeds. J. Mol. Liq. 2016, 219, 359–367. [Google Scholar] [CrossRef]
- Reddy, M.S.; Sivaramakrishna, L.; Reddy, A.V. The use of an agricultural waste material, Jujuba seeds for the removal of anionic dye (Congo red) from aqueous medium. J. Hazard. Mater. 2012, 203–204, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Arlorio, M.; Coïsson, J.; Travaglia, F.; Varsaldi, F.; Miglio, G.; Lombardi, G.; Martelli, A. Antioxidant and biological activity of phenolic pigments from Theobroma cacao hulls extracted with supercritical CO2. Food Res. Int. 2005, 38, 1009–1014. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa bean shell waste valorisation; extraction from lab to pilot-scale cavitational reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef] [PubMed]
- González-Alejo, F.A.; Barajas-Fernández, J.; Olán-Acosta, M.D.L.Á.; Lagunes-Gálvez, L.M.; García-Alamilla, P. Supercritical Fluid Extraction of Fat and Caffeine with Theobromine Retention in the Cocoa Shell. Processes 2019, 7, 385. [Google Scholar] [CrossRef] [Green Version]
- Massoudinejad, M.; Ghaderpoori, M.; Shahsavani, A.; Amini, M.M. Adsorption of fluoride over a metal organic framework Uio-66 functionalized with amine groups and optimization with response surface methodology. J. Mol. Liq. 2016, 221, 279–286. [Google Scholar] [CrossRef]
- Montgomery, D.C. Response surface methods and designs. In Design and Analysis of Experiments; Wiley: New York, NY, USA, 2001; p. 455. [Google Scholar]
- Lafi, R.; Montasser, I.; Hafiane, A. Adsorption of congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt. Sci. Technol. 2019, 37, 160–181. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Lozada, G.M.C.; Soria, A.O.; Jaramillo, O.Y.L. Espectroscopia de Infrarrojo. In Conceptos y Problemas; Primera edición; Universidad Autonóma Metropolitana, Unidad Xochimilco: Ciudad de México, México, 2013. [Google Scholar]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.; Bedin, K.C.; Beltrame, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]
- Marsiglia, D.E.; Ojeda, K.A.; Ramírez, M.C.; Sánchez, E. Pectina extraction from cocoa pod husk (Theobroma cacao L.) by hydrolysis with citric and acetic acid. Int. J. Chem. Tech. Res. 2016, 9, 497–507. [Google Scholar]
- Gutiérrez-Pulido, H.; de la Vara-Salazar, R.N. Análisis Y Diseño De Experimentos; Mc Graw Hill Interamericana: Mexido, Mexico, 2008. [Google Scholar]
- Iqbal, M.; Iqbal, N.; Bhatti, I.A.; Ahmad, N.; Zahid, M. Response surface methodology application in optimization of cadmium adsorption by shoe waste: A good option of waste mitigation by waste. Ecol. Eng. 2016, 88, 265–275. [Google Scholar] [CrossRef]
- Zhang, Z.; Moghaddam, L.; O’Hara, I.M.; Doherty, W.O. Congo Red adsorption by ball-milled sugarcane bagasse. Chem. Eng. J. 2011, 178, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Kumari, H.J.; Krishnamoorthy, P.; Arumugam, T.; Radhakrishnan, S.; Vasudevan, D. An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: A novel low cost adsorbent. Int. J. Biol. Macromol. 2017, 96, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Mittal, J.; Malviya, A.; Gupta, V. Adsorptive removal of hazardous anionic dye “Congo red” from wastewater using waste materials and recovery by desorption. J. Colloid Interface Sci. 2009, 340, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Li, C.; Yang, H.; Ye, Z.; Xu, H. Spent mushroom: A new low-cost adsorbent for removal of Congo Red from aqueous solutions. Desalination Water Treat. 2011, 27, 319–326. [Google Scholar] [CrossRef]
- Jesudos, H.N.R.; Kumar, J.S.; Kamyab, H.; Jennifa, S.J.A.; Al-Khashman, A.A.; Kuslu, Y.; Ene, A.; Kumar, B.S. Modern enabling techniques and adsorbents basd dye removal with sustainability concerns in textile industrial sector—A comprehensive review. J. Clean. Prod. 2020, 272, 122636. [Google Scholar] [CrossRef]



| Adimensional Factor | Name | Low Level | Central Level | High Level | Coded Low | Coded Central | Coded High |
|---|---|---|---|---|---|---|---|
| X1 | Dye [mg L−1] | 60 | 80 | 100 | −1 | 0 | +1 |
| X2 | Bioadsorbent (g) | 0.09 | 0.12 | 0.15 | −1 | 0 | +1 |
| X3 | pH | 5 | 7 | 9 | −1 | 0 | +1 |
| X4 | Time (h) | 12 | 20 | 28 | −1 | 0 | +1 |
| Run | Coded Variables | Natural Variables | % Adsorption | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Dye (mg L−1) | Bioadsorbent (g) | pH | Time (h) | ||
| 1 | −1 | −1 | −1 | −1 | 60 | 0.09 | 5 | 12 | 85.32 |
| 2 | −1 | −1 | −1 | 1 | 60 | 0.09 | 5 | 28 | 87.82 |
| 3 | −1 | −1 | 1 | −1 | 60 | 0.09 | 9 | 12 | 78.48 |
| 4 | −1 | −1 | 1 | 1 | 60 | 0.09 | 9 | 28 | 85.43 |
| 5 | −1 | 1 | −1 | −1 | 60 | 0.15 | 5 | 12 | 85.22 |
| 6 | −1 | 1 | −1 | 1 | 60 | 0.15 | 5 | 28 | 87.92 |
| 7 | −1 | 1 | 1 | −1 | 60 | 0.15 | 9 | 12 | 84.57 |
| 8 | −1 | 1 | 1 | 1 | 60 | 0.15 | 9 | 28 | 85.32 |
| 9 | 1 | −1 | −1 | −1 | 100 | 0.09 | 5 | 12 | 80.47 |
| 10 | 1 | −1 | −1 | 1 | 100 | 0.09 | 5 | 28 | 79.96 |
| 11 | 1 | −1 | 1 | −1 | 100 | 0.09 | 9 | 12 | 74.29 |
| 12 | 1 | −1 | 1 | 1 | 100 | 0.09 | 9 | 28 | 77.41 |
| 13 | 1 | 1 | −1 | −1 | 100 | 0.15 | 5 | 12 | 80.90 |
| 14 | 1 | 1 | −1 | 1 | 100 | 0.15 | 5 | 28 | 83.42 |
| 15 | 1 | 1 | 1 | −1 | 100 | 0.15 | 9 | 12 | 84.01 |
| 16 | 1 | 1 | 1 | 1 | 100 | 0.15 | 9 | 28 | 79.74 |
| 17 | −2 | 0 | 0 | 0 | 40 | 0.12 | 7 | 20 | 85.56 |
| 18 | 2 | 0 | 0 | 0 | 120 | 0.12 | 7 | 20 | 79.79 |
| 19 | 0 | −2 | 0 | 0 | 80 | 0.06 | 7 | 20 | 77.84 |
| 20 | 0 | 2 | 0 | 0 | 80 | 0.18 | 7 | 20 | 81.34 |
| 21 | 0 | 0 | −2 | 0 | 80 | 0.12 | 3 | 20 | 87.58 |
| 22 | 0 | 0 | 2 | 0 | 80 | 0.12 | 11 | 20 | 85.74 |
| 23 | 0 | 0 | 0 | −2 | 80 | 0.12 | 7 | 4 | 80.67 |
| 24 | 0 | 0 | 0 | 2 | 80 | 0.12 | 7 | 36 | 89.86 |
| 25 | 0 | 0 | 0 | 0 | 80 | 0.12 | 7 | 20 | 84.76 |
| 26 | 0 | 0 | 0 | 0 | 80 | 0.12 | 7 | 20 | 81.07 |
| 27 | 0 | 0 | 0 | 0 | 80 | 0.12 | 7 | 20 | 83.43 |
| 28 | 0 | 0 | 0 | 0 | 80 | 0.12 | 7 | 20 | 84.72 |
| 29 | 0 | 0 | 0 | 0 | 80 | 0.12 | 7 | 20 | 83.50 |
| 30 | 0 | 0 | 0 | 0 | 80 | 0.12 | 7 | 20 | 84.33 |
| Parameters | Estimated Coefficient | Standard Error | t Test Statistics | p-Value |
|---|---|---|---|---|
| Constant | 83.704 | 0.6608 | 126.67 | 9.77 × 10−27 |
| X1 | −2.1421 | 0.3815 | −5.6147 | 3.09 × 10−5 * |
| X2 | 1.2054 | 0.3815 | 3.1594 | 0.0057 * |
| X3 | −1.0612 | 0.3815 | −2.7816 | 0.0127 * |
| X4 | 1.3388 | 0.3815 | 3.5091 | 0.0026 * |
| X1X2 | 0.6226 | 0.4672 | 1.3325 | 0.2002 |
| X1X3 | 0.2001 | 0.4672 | 0.4283 | 0.6737 |
| X1X4 | −0.7536 | 0.4672 | −1.6129 | 0.1251 |
| X2X3 | 0.8845 | 0.4672 | 1.893 | 0.0755 |
| X2X4 | −0.6461 | 0.4672 | −1.3829 | 0.1846 |
| X3X4 | −0.0404 | 0.4672 | −0.0865 | 0.9320 |
| X12 | −0.4283 | 0.3439 | −1.2456 | 0.2298 |
| X22 | −1.1998 | 0.3439 | −3.4888 | 0.0028 * |
| X32 | 0.5667 | 0.3439 | 1.648 | 0.1177 |
| X42 | 0.2179 | 0.3439 | 0.6337 | 0.5346 |
| Sum of Squares | Degrees of Freedom | Average Squares | F | p-Value | |
|---|---|---|---|---|---|
| Total | 371.91 | 31 | 11.997 | ||
| Model | 312.52 | 14 | 22.323 | 6.3899 | 0.0002 |
| 215.05 | 4 | 53.762 | 15.389 | 1.71 × 105 * |
| 97.471 | 10 | 9.7471 | 2.7901 | 0.0302 * |
| Residue | 59.389 | 17 | 3.4935 | ||
| 49.338 | 10 | 4.9338 | 3.4361 | 0.0574 |
| 10.051 | 7 | 1.4359 |
| Factor | Low | High | Optimal |
|---|---|---|---|
| Dye concentration | −2.0 | 2.0 | −2.0 |
| Bioadsorbent | −2.0 | 2.0 | 0.79 |
| pH | −2.0 | 2.0 | −2.0 |
| Time | −2.0 | 2.0 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Arellano, G.; Barajas-Fernández, J.; García-Alamilla, R.; Lagunes-Gálvez, L.M.; Lara-Rivera, A.H.; García-Alamilla, P. Evaluation of Cocoa Beans Shell Powder as a Bioadsorbent of Congo Red Dye Aqueous Solutions. Materials 2021, 14, 2763. https://doi.org/10.3390/ma14112763
Rodríguez-Arellano G, Barajas-Fernández J, García-Alamilla R, Lagunes-Gálvez LM, Lara-Rivera AH, García-Alamilla P. Evaluation of Cocoa Beans Shell Powder as a Bioadsorbent of Congo Red Dye Aqueous Solutions. Materials. 2021; 14(11):2763. https://doi.org/10.3390/ma14112763
Chicago/Turabian StyleRodríguez-Arellano, Gabriela, Juan Barajas-Fernández, Ricardo García-Alamilla, Laura Mercedes Lagunes-Gálvez, Antonio Hilario Lara-Rivera, and Pedro García-Alamilla. 2021. "Evaluation of Cocoa Beans Shell Powder as a Bioadsorbent of Congo Red Dye Aqueous Solutions" Materials 14, no. 11: 2763. https://doi.org/10.3390/ma14112763
APA StyleRodríguez-Arellano, G., Barajas-Fernández, J., García-Alamilla, R., Lagunes-Gálvez, L. M., Lara-Rivera, A. H., & García-Alamilla, P. (2021). Evaluation of Cocoa Beans Shell Powder as a Bioadsorbent of Congo Red Dye Aqueous Solutions. Materials, 14(11), 2763. https://doi.org/10.3390/ma14112763