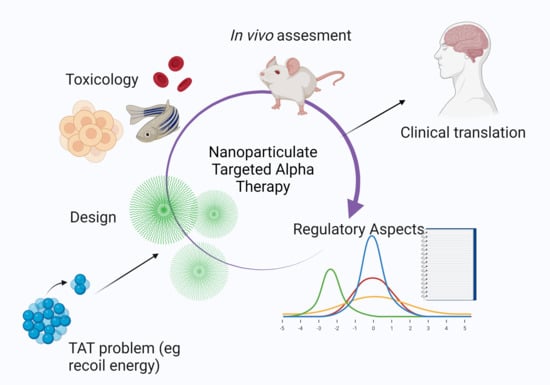
Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy
Abstract
:1. Introduction
2. Current Challenges Using Targeted Alpha Therapy
2.1. Production Aspect and Limitations of Physical Half-Life
2.2. Chemistry Constraints
2.3. Pharmacokinetic Behavior
3. Radiolabeled Nanoparticle-Based Systems Applicable to TAT
4. Pitfalls for Bench-to-Bedside Translation
4.1. Large-Scale Manufacturing
4.2. Size of Nano-Constructs
4.3. Regulatory Challenges
4.4. Toxicity
5. Toxicity and Tolerability of Nanoparticulate Systems
6. The Way Forward?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Majkowska-Pilip, A.; Gaweda, W.; Zelchowska-Matysiak, K.Z.; Wawrowicz, K.; Bilewicz, A. Nanoparticles in targeted alpha therapy. Nanomaterial 2020, 10, 1366. [Google Scholar] [CrossRef]
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. Part A 2019, 107A, 251–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleynhans, J.; Grobler, A.F.; Ebenhan, T.; Sathekge, M.M.; Zeevaart, J.R. Radiopharmaceutical enhancement by drug delivery systems: A review. J. Control. Release 2018, 287, 177–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roscher, M.; Bakos, G.; Benesova, M. Atomic nanogenerators in targeted alpha therapies: Curie’s legacy in modern cancer management. Pharmaceutics 2020, 13, 76. [Google Scholar] [CrossRef]
- Mittra, E.S. Neuroendocrine Tumor Therapy: 177Lu-DOTATATE. AJR Am. J. Roentgenol. 2019, 211, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Lewington, V.; Shore, N.; Kratochwil, C.; Levy, M.; Linden, O.; Noordzij, W.; Park, J.; Saad, F.; Targeted Alpha Therapy Working Group. Targeted alpha therapy, an emerging class of cancer agents: A review. JAMA Oncol. 2018, 4, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Liberal, F.D.C.G.; O’Sullivan, J.M.; Mcmahon, S.J.; Prise, K.M. Targeted alpha therapy: Current clinical applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Behesthi, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An overview of targeted alpha theranostics with 225 Actinium and 213 Bismuth. Curr. Radiopharm. 2018, 11, 200–208. [Google Scholar] [CrossRef]
- Bruchertseifer, F.; Kellerbauer, A.; Malmbeck, R.; Morgenstern, A. Targeted alpha therapy with bismuth-213 and actinium-225: Meeting the future demand. J. Label. Comp. Radiopharm. 2019, 62, 794–802. [Google Scholar] [CrossRef]
- Engle, J.W. The production of ac-225. Curr. Radiopharm. 2018, 11, 173–179. [Google Scholar] [CrossRef]
- Garashchenko, B.L.; Korsakova, V.A.; Yakovlev, R.Y. Radiopharmaceuticals based on alpha emitters: Preparation, properties and application. Phys. At. Nucl. 2018, 81, 1515–1525. [Google Scholar] [CrossRef]
- Lindegren, S.; Albertsson, P.; Back, T.; Jensen, H.; Palm, S.; Anaheim, E. Realizing clinical trials with astatine-211: The chemistry infrastructure. Cancer Biother. Radiopharm. 2020, 35, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Harman, K.B.; Hamlin, D.K.; Wilbour, D.S.; Wilson, L.J. 211ACl@US-Tube nanocapsules: A new concept in radiotherapeutic-agent design. Small 2007, 3, 1496–1499. [Google Scholar] [CrossRef]
- Kucka, J.; Hruby, M.; Konak, C.; Kozempel, J.; Lebeda, O. Astatination of nanoparticles containing sliver as possible carriers of 211 At. Appl. Radiat. Isot. 2006, 64, 201–206. [Google Scholar] [CrossRef]
- Almasi, T.; Gholipour, N.; Akhlagi, M.; Keirabadi, A.M.; Mazidi, S.M.; Hosseini, S.H.; Geramifar, P.; Beiki, D.; Rostampour, N.; Shahbazi-Gahrouei, D. Functionalised and acetylated PAMAM dendrimer-coated iron oxide nanoparticles as PET/MR dual-modal imaging agents. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–13. [Google Scholar] [CrossRef]
- Al-Qahtani, M.; Malki, Y.A.; Mutwali, H.; Helal-Neto, E.; Santos-Oliveira, R. Ga-68 nanoparticles and ultra-small nanoparticles: Next generation of PET radiopharmaceuticals? Curr. Radiopharm. 2018, 11, 123–129. [Google Scholar] [CrossRef]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, M.; Fassbender, M.E.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-emitters and targeted alpha therapy in oncology: From basic science to clinical investigations. Target Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Dziawer, L.; Majkowska-pili, A.; Gawel, D.; Godlewska, M.; Pruszynski, M.; Jastrezebski, J.; Was, B.; Bilewicz, A. Trastuzumab-modified gold nanoparticles labeled with 211At as a prospective tool for local treatment of HER-2 positive breast cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [Green Version]
- Dziawer, L.; Kozminzki, P.; Meczynska-Wielgosz, S.; Pruzynski, M.; Lyczko, M.; Was, B.; Celichowski, G.; Grobelny, J.; Jastrzębski, J.; Bilewicz, A. Gold nanoparticle bioconjuages labelled with 221At for targeted alpha therapy. RSC Adv. 2017, 65, 41024. [Google Scholar] [CrossRef] [Green Version]
- Woodward, J.; Kennel, S.J.; Stucky, A.; Osborne, D.; Wall, J.; Rondione, A.J.; Standaert, R.F.; Mirzadeh, S. LaPO4 nanoparticles doped with actinium-225 that partially sequester daughter radionuclides. Bioconjug. Chem. 2011, 22, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Salvanou, E.A.; Stellas, D.; Tsoukalas, C.; Mavroidi, B.; Paravatou-Petsotas, M.; Kalogeropoulos, N.; Xanthopoulos, S.; Denat, F.; Laurent, G.; Bassi, R.; et al. A proof-of-concept study on the therapeutic potential of Au nanoparticles radiolabeled with the alpha-emitter actinium-225. Pharmaceutics 2020, 12, 188. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-Y.; Seideman, J.; Sofou, S. Enhanced loading efficiency and retention of 225Ac in rigid liposomes for potential targeted therapy of micrometastases. Bioconjug. Chem. 2008, 19, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Toro-Gonzalez, M.; Dame, A.N.; Mirzadeh, S.; Rojas, J.V. Gadolinium vanadate nanocrystals as carriers of α-emitters (225Ac, 227Th) and contrast agents. J. App. Phys. 2019, 125, 214901. [Google Scholar] [CrossRef]
- De Kruif, R.M.; Drost, K.; Thijssen, L.; Morgensern, A.; Bruchertseifer, F.; Lathouwers, D.; Wolterbeek, H.A.T.; Denkova, A.G. Improved 225Ac daughter retention in InPO4 containing polymersomes. App. Radiat. Isot. 2017, 128, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sofou, S.; Thomas, J.L.; Lin, H.-Y.; McDevitt, M.R.; Scheinberg, D.A.; Sgouros, G. Engineered liposomes for potential alpha-particle therapy of metastatic cancer. J. Nucl. Med. 2005, 45, 253–260. [Google Scholar]
- Sofou, S.; Kappel, B.J.; Jaggi, J.S.; McDevitt, M.R.; Scheinberg, D.A.; Sgourous, G. Enhanced retention of alpha-particle emitting daughters of actinium-225 by liposome carriers. Bioconjug. Chem. 2008, 18, 201–2067. [Google Scholar] [CrossRef] [Green Version]
- Brandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgernstern, A.; Sofou, S. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular alpha particle therapy of cancer. J. Nucl. Med. 2014, 55, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; De Kruijff, R.M.; Rol, A.; Thijssen, L.; Mendes, E.; Morgenstern, A.; Bruchertseifer, F.; Stuart, M.C.A.; Wolterbeek, H.T.; Denkova, A.G. Retention studies of recoiling daughter nuclides of 225Ac in polymer vesicles. Appl. Radiat. Isot. 2014, 85, 45–53. [Google Scholar] [CrossRef]
- Sukthankar, P.; Avial, L.A.; Whitaker, S.K.; Iwamoto, T.; Morgenstern, A.; Apostolidis, C.; Liu, K.; Hanzlik, R.P.; Dadachova, E.; Tomich, J.M. Branched amphiphilic peptide capsules: Cellular uptake and retention of encapsulated solutes. Biochim. Biophys. Acta BBA Biomembr. 2014, 1838, 2296–2305. [Google Scholar] [CrossRef]
- Sattiraju, A.; Xiong, X.; Pandaya, D.N.; Wadas, T.J.; Xuan, A.; Sun, Y.; Jung, Y.; Sai, K.S.S.; Dorsey, J.F.; Li, K.C.; et al. Alpha particle enhanced blood brain/tumour barrier permeabilization in glioblastomas using integra alpha-v beta-3 targeted liposomes. Mol. Cancer Ther. 2017, 16, 2191–2220. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, G.; Schoultz, B.W.; Michaelsen, T.E.; Bruland, O.S.; Larsen, R.H. Sterically stabilized liposomes as a carrier for alpha emitting radium and actinium radionuclides. Nucl. Med. Biol. 2004, 31, 441–449. [Google Scholar] [CrossRef]
- Toro-Gonzalez, M.; Copping, R.; Mirzadeh, S.; Rojas, J.V. Multifunctional GdVo4: Eu core-shell nanoparticles containing 225Ac for targeted alpha therapy and molecular imaging. J. Mater. Chem. B 2018, 47, 7985–7997. [Google Scholar] [CrossRef] [PubMed]
- Jonasdottir, T.J.; Fisher, D.R.; Borrabeak, J.; Bruland, O.S.; Larsen, R.H. First in vivo evaluation of liposome-encapsulated 223R as a potential alpha-particle-emitting cancer therapeutic agent. Anticancer Res. 2006, 26, 2841–2848. [Google Scholar]
- Reissig, F.; Zarschler, K.; Hubner, R.; Pietzsch, H.-J.; Kopka, K.; Mamat, C. Sub-10 nm radiolabeled barium sulfate nanoparticles as carriers for theranostic applications and targeted alpha therapy. Chem. Open 2020, 9, 796. [Google Scholar] [CrossRef]
- Piotrowska, A.; Meczynska-Wielgosz, S.; Majkowska-Pilip, A.; Kozminski, P.; Wojciuk, G.; Cedrowska, E.; Bruchterseifer, F.; Morgenstern, A.; Kruszewski, M.; Bilewicz, A. Nanozeolite bioconjugates labeled with radium-223 for targeted alpha therapy. Nucl. Med. Biol. 2017, 47, 10–18. [Google Scholar] [CrossRef]
- Mokhodoeva, O.; Vlk, M.; Malkova, E.; Kukleva, E.; Micolova, P.; Stamberg, K.; Slouf, M.; Dzhenloda, R.; Kozempel, J. Study of radium-223 uptake mechanism by Fe3O4 nanoparticles: Toward new prospective theranostics SPIONS. J. Nanopart. Res. 2016, 18, 301. [Google Scholar] [CrossRef]
- Du, A.L.; Mougin-Degraef, M.; Botosoa, E.; Rauscher, A.; Chauvet, F.; Barbet, J.; Montavon, G. In vivo 212Pb/212Bi generator using indium-DTPA-tagged liposomes. Radiochem. Acta 2011, 99, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, G.; Schoultz, B.W.; Hoff, P.; Larsen, R.H. Potential in vivo generator for alpha-particle therapy with 212Bi: Presentation of a system to minimize the escape of daughter nuclide after decay of 212Pb to 212Bi. Radiochim. Acta 2003, 91, 109–113. [Google Scholar] [CrossRef]
- Severin, A.V.; Orlova, M.A.; Shalamova, E.S.; Egorov, A.V.; Sirotin, M.A. Nanohydroxyapaptie and its textures as potential carriers of promising short-lived lead isotopes. Russ. Chem. Bull. Int. Ed. 2019, 68, 2197–2204. [Google Scholar] [CrossRef]
- Diener, M.D.; Alford, J.M.; Kennel, S.J.; Mirzadeh, S. 212Pb@60 and its water-soluble derivatives: Synthesis, stability and suitability for radioimmunotherapy. J. Am. Chem. Soc. 2007, 129, 5131–5138. [Google Scholar] [CrossRef] [PubMed]
- Guerard, F.; Gestin, J.F.; Brechbiel, M.W. Production of [211At]Astinated radiopharmaceuticals and applications in targeted alpha particle therapy. Cancer Biother. Radiopharm. 2013, 28, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Gaweda, W.; Pruzynski, M.; Cedrowska, E.; Rodak, M.; Majokwska-Pilip, A.; Gawel, D.; Bruchertseifer, F.; Morgenstern, A.; Bilewicz, A. Traztuzumab modified barium ferrite magnetic nanoparticles labeled with radium-223: A new potential radiobioconjuagte for alpha radioimmunotherapy. Nanomaterials 2020, 10, 2067. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.A.; Bagheri, R.; Mosafer, J.; Baradaran, B.; Hashemzaei, M.; Baghbanzadeh, A.; De la Guardia, M.; Mokhtarzadeh, A. Recent advances on thermosensitive and pH-sensitive liposomes employed in controlled release. J. Control. Release 2019, 315, 1–22. [Google Scholar] [CrossRef]
- Xiang, B.; Dong, D.-W.; Shi, N.-Q.; Gao, W.; Yang, Z.-Z.; Cui, Y.; Gao, D.-Y.; Qi, X.-R. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials 2013, 34, 6976–6991. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Sindhwani, S.; Chan, W.C.W. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Gibson, N.; Holzwarth, U.; Abbas, U.H.K.; Simoneli, F.; Kozempel, J.; Cydzik, I.; Cotogno, G.; Bulgheroni, A.; Gilliland, D.; Ponti, J.; et al. Radiolabelling of engineered nanoparticles for in vitro and in vivo tracing applications using cyclotron accelerators. Arch. Toxicol. 2011, 85, 751–753. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Q.; Zeng, J.; Gu, Z.; Gao, M. Radiolabeling nanomaterials for multimodality imaging: New insights into nuclear medicine and cancer diagnosis. Biomaterials 2020, 228, 119553. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; De Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 5, 3355–3423. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, L.; Chen, H.; Wang, L.; Liu, T.; Yeh, J.; Lu, G.; Yang, L. Delivery of therapeutic radioisotopes using nanoparticle platforms: Potential benefit in systemic radiation therapy. Nanotechnol. Sci. Appl. 2010, 3, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in targeted alpha-particle therapy. What we learned about recoils release from in vivo generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, A.; Leszczuk, E.; Bruchertseifer, F.; Morgernstern, A.; Bilewicz, A. Functionalized NaA nanozeolites labeled with 224,225Ra for targeted alpha therapy. J. Nanopart. Res. 2013, 15, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzwarth, U.; Jimenez, I.O.; Calzolai, L. A random walk approach to estimate the confinement of α-particle emitters in nanoparticles for targeted radionuclide therapy. EJNMMI Radiopharm. Chem. 2018, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The clinical translation of organic nanomaterials for cancer therapy: A focus on polymeric nanoparticles, micelles, liposomes and exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Sainz, V.; Connoit, J.; Matos, A.I.; Peres, C.; Zupancic, E.; Moura, L.; Sliva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef]
- Steichen, S.D.; Caldorera-Moore, M.; Peppas, N.A. A review of current nanoparticles and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013, 48, 416–427. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties and regulatory issues. Front. Chem. 2018, 6L360. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; De Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9790. [Google Scholar] [CrossRef]
- Jones, A.D.; Mi, G.; Webster, T.J. A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol. 2019, 37, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decristoforo, C.; Neels, O.; Patt, M. Emerging radionuclides in a regulatory framework for medicinal products–how do they fit. Front. Nucl. Med. 2021, 8, 678452. [Google Scholar] [CrossRef] [PubMed]
- Keck, S.M.; Müller, R.H. Nanotoxicological classification system (NCS)–A guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur. J. Pharm. Biopharm. 2013, 84, 445–448. [Google Scholar] [CrossRef]
- Ajdary, M.; Moosavi, M.A.; Rahmati, M.; Falahati, M.; Mahboubi, M.; Mandegary, A.; Jangjoo, S.; Mohammadinejad, R.; Varma, R.S. Health concerns of various nanoparticles: A review of their in vitro and in vivo toxicity. Nanomaterials 2018, 8, 634. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.S.; Frangioni, J.V. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 291–310. [Google Scholar] [CrossRef]
- Gicheva, G.; Yordanov, G. Environmental impact of nanomaterials. In Colloid and Interface for Nanotechnology; Chapter 2: Environmental Impact of Nanomaterials; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Drude, N.; Tienken, L.; Mottaghy, F.M. Theranostic and nanotheranostic probes in nuclear medicine. Methods 2017, 130, 14–22. [Google Scholar] [CrossRef]
- Pandit-Taskar, N. Targeted radioimmunotherapy and theranostics with alpha emitters. J. Med. Imaging Radiat. Sci. 2019, 50, S41–S44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, S.; Shiga, T.; Tamaki, N. Clinical perspectives of theranostics. Molecules 2018, 26, 2232. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Rudzinska, M.; Leporatti, S.; Anissimov, Y.; Zamyatnin, A.A. Smart nanotheranostics responsive to pathological stimuli. Front. Bioeng. Biotechnol. 2020, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, M.; Eberlein, U. Targeted alpha-particle therapy: Imaging, dosimetry and radiation protection. Ann. ICRP 2018, 47, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical properties of nanomaterials: Implication in associated toxic manifestations. BioMed Res. Int. 2014, 2014, 498420. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, R.S. Therapeutic products regulating drugs and medical devices. In International Handbook on Regulating Nanotechnologies; Hodge, G.A., Bowman, D.M., Maynard, A.D., Eds.; Edward Elgar Publishing Limited: Northampton, MA, USA, 2010; pp. 291–320. [Google Scholar] [CrossRef]
- Krolicki, L.; Bruchertseifer, F.; Kunikowska, J.; Koziara, H.; Krolicki, B.; Jakuncinski, M.; Pawlak, D.; Apostolidis, C.; Mirzadeh, S.; Rola, R.; et al. Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1636–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Ehlerding, E.B.; Cai, W. Theranostic nanoparticles. J. Nucl. Med. 2014, 55, 1919–1922. [Google Scholar] [CrossRef] [Green Version]





| System | Benefit of Nanotechnology | Labeling Conditions | Findings | Ref. |
|---|---|---|---|---|
| Astatine-211 | ||||
| Ultrashort carbon nanotubes | Containment of astatine-211 in a nano-particle that allows binding to targeting molecule | Ultrashort carbon nanotubes were incubated for 10 min with astatine-211 (211At−) solution at room temperature in both H2O and methanol. | Demonstrated entrapment of astatine-211 inside nanotube and in vitro stability. | [14] |
| Silver containing nanoparticles coated with poly-ethylene oxide | Containment of astatine-211 in a nanoparticle that allows binding to targeting molecule | A solution of sliver nanoparticles was added to astatine-211 as distilled from the target and eluted in methanol, and left to react for 15 min. | Labeling yields in different conditions were found to be between 50% and 97%. No in vitro studies performed. | [15] |
| Trastuzumab-gold nanoparticles labeled with astatine-211 | Containment of astatine-211 in a nanoparticle that allows binding to targeting molecule | Astatine-211 (211At−) was added to AuNP-S-PEG-trastuzumab bioconjugate and stirred for 1 h at room temperature. This method made use of adsorption. | Demonstrated in vitro stability as well as high affinity and cytotoxicity towards HER-2-overexpressing SKOV-2 cells. | [19] |
| Gold nanoparticle for astatine-211 delivery | Labeling of biomolecules with astatine-211 by applying gold nanoparticles | Prepared by adsorption of astatine-211 (211At−) on already produced and analyzed gold nanoparticles (in deionized water). Process took ± 1 h. | Demonstrated in vitro stability in human fluid and cerebrospinal fluid for 24 h. Hight cytotoxic effect on in vitro glioma cells. | [20] |
| Actinium-225 | ||||
| Lanthanum phosphate nanoparticles | Enhanced retention of actinium-225 and decay daughters | Actinium-225 was incorporated during production in the form of [225Ac]AcCl3. Process took <4 h and nanoparticles were made by precipitation. | mAb 201B—targeting mouse lung endothelium | [21] |
| Gold nanoparticle [225Ac]Ac-AU@TADOTAGA | Brachytherapy IV and IT for injectable radiopharmaceutical | Au@TADOTAGA gold particles and [225Ac]AcCl3 were incubated at 70 °C for 30 min. The nanoparticles are designed with a chelator to incorporate the metal radionuclide. | U87MG tumor-bearing mice—controls inadequate | [22] |
| Antibody conjugated liposomes | Increase delivery of actinium-225 to target tumor | Active loading of actinium-225 with ionophores. Incubation times differed from 15 to 60 min with DOTA inside the liposome as chelator. Unlabeled actinium-225 was removed with DTPA from the mixture. | Actiniuum-225 retention as high as 81% ± 7% was obtained. No in vitro tests performed. | [23] |
| Core–shell Gd0.8Eu0.2VO4 NPs doped with actinium-225 | Enhanced retention of actinium-225 and decay daughters | The actinium-225 was incorporated from the start of the synthesis and crystallization of nanoparticles. The radionuclide is in cationic chemical form. | Luminescence and magnetic functionalities demonstrated and radionuclide retention. | [24] |
| InPO4 containing polymersomes | Enhanced retention of actinium-225 and decay daughters | Actinium-225 (dry actinium-225 nitrate) was incorporated in an ionophore film (dissolving the actinium-225 in this solution), this was incubated with InPO4 nanoparticles for 1 h. | Increased retention of francium-221 and bismuth-213 | [25] |
| Multivesicular liposomes conjugated to HER2/neu antibody | Enhanced retention of actinium-225 and decay daughters | Actinium-225 was entrapped passively in lipids resuspended with PBS containing [225Ac]Ac-DOTA. Note that direct labeling of antibodies will cause damage since acintium-225-DOTA labeling happens at higher temperatures. DOTA act as chelator for the metal radionuclide. | HER2/neu antibody targeting ovarian cancer cells (SKOV3-NMP2) deliver higher fractions of generated alpha particles | [26,27] |
| PEGylated liposomes targeted with antihuman PSMA J591 antibody or A10 PSMA aptamer | Optimizing biodistribution due to the ease of modification of liposomes | Actinium-225 was loaded in preformed DOTA-containing liposomes mediated by ionophores. Efficient loading took place at 1 h and a temperature of 65 °C. DOTA act as chelators for the metal radionuclide. | J591-labeled liposomes demonstrated higher specific binding to all cell lines compared with A10 aptamer-labeled liposomes | [28] |
| Actinium-225 loaded in polymersomes | Enhanced retention of actinium-225 and decay daughters | Polymersomes with encapsulated DTPA was incubated for 30 min with actinium-225 ([225Ac]AcCl3). | Displays little loss of daughters if no recoil is present. | [29] |
| Peptide-based nano-assembly | Enhanced retention of actinium-225 and decay daughters | The peptides where mixed and dried. After drying, peptides were incubated with actinium-225 and DOTA for 2 h in an ammonium acetate buffer. | Branched amphiphilic peptide capsules that self-assemble and retainment of bismuth-213 in vivo. | [30] |
| Actinium-225-labeled αvβ3-specific liposomes | Targeting alpha therapy to the blood–brain barrier | The prepared liposomes were incubated with actinium-225 ([225Ac]AcCl3) at 70 °C for 50 min. | Demonstrated in vivo therapeutic efficacy in orthotopic glioblastoma | [31] |
| Sterically stabilized liposomes coated with folate-F(ab’)2 | Enhanced retention of actinium and radium and decay daughters | Prepared by loading preformed liposomes with a Ca-ionophore. Incubation with the radionuclide was done for 30 min at 65 °C. Quenching of the reaction was done by adding EDTA and PBS to the mixture. | Radionuclide-loaded liposomes demonstrated serum stability in vitro | [32] |
| Thorium-227 | ||||
| Core–shell Gd0.8Eu0.2VO4 NPs doped with thorium-227 | Enhanced retention of thorium-227 and decay daughters | The thorium-227 was incorporated from the start of the synthesis and crystallization of nanoparticles. | Luminescence and magnetic functionalities demonstrated and radionuclide retention. | [33] |
| Radium-223/radium-224 | ||||
| Combination chemotherapeutic radium-223 liposomes | Combining chemotherapeutic agent (doxorubicin) with radium-223 therapy | A calcium ionophore was used to entrap radium-223 in preformed liposomes. Entrapment took ±1 h. Radium-223 was added in a solution of sucrose and HEPES. | Liposomal radium-223 was stable in vivo and demonstrated potential. | [34] |
| Functionalized nanoparticles based on BaSO4 to allow binding of radium-223 to targeting molecule | Allow binding of radium-224 to targeting molecules through a functionalized liposome | Nanoparticles are obtained after reprecipitation [224Ra]Ra(NO3)2. Precipitation takes 15 min, after which particles are centrifuged for 15 min. | Show stability of >90% regarding radiometal release from BaSO4 matrix. Not evaluated in vivo. | [35] |
| Nanzeolite particles conjugated to Substance P labeled with radium-223 | Allow binding of radium-223 to targeting molecule through a functionalized nanoparticle | Nanozeolite particles were sonificated in a radium-223 solution for 15 min, stirred for another 2 h, and centrifuged for 10 min. The 223Ra(NO3)2 was used to produce 223Ra2+ cations. | Demonstrated being a viable technology retaining radium-223 for up to 6 days in vitro and retaining 90–95% of daughter decay products. | [36] |
| SPIONs to drive the therapeutic delivery of radium-223 by magnetic field gradients | Allow binding of radium-223 to magnetic particle that can be targeted | Evaporate radium-223 solution (223Ra(NO3)2) to dryness. Expose nanoparticles for 30–60 min to radium-223 for labeling. | Not tested in vivo, it demonstrated good stability in vitro and high radiolabeling yields. | [37] |
| Lead-212 | ||||
| Lead-212 internalized into surface-DTPA liposomes | To use the parent radionuclide lead-212 to deliver bismuth-212 to the target area. | A two-step preparation process: label surface-DTPA liposome with indium and then incubate with lead-212, which naturally passes through the liposome and is entrapped by internal DTPA. | A novel method for entrapment of 2–3 lead atoms per liposome with a yield of 75%. No in vitro testing performed. | [38] |
| Sterically stabilized liposomes | Minimize the escape of daughter bismuth-212 after decay from lead-212. | Ionophore-mediated loading of lead-212 into preformed liposomes (incubated for 30 min). | Retention of lead-212 and bismuth 212 was 95% after incubation for 20 h at 37 °C in serum. | [39] |
| Sorption and co-crystallization of lead-212 with nanohydroxyapatite | Transporting lead-212 to the targeting tumor. | Co-crystallization of the lead-212 during nanohydroxyapatite production. | Various parameters in production of nanohydroxyapatite with lead-212 was investigated. No in vitro testing was performed. | [40] |
| Fullerenes containing lead-212 | Reduce myelotoxicity resulting from accumulation of lead-212 in the bone marrow. | Incorporation into fullerene by recoil of polonium-218 parent. | Lead-212 fullerene resulted in inhibition of accumulation of lead-212 to the bone in vivo. | [41] |
| NP Parameter | Status | Current Guidelines |
|---|---|---|
Size | Toxicity influenced by surface-to-volume ratio. | NPs < 50 nm reach tissue faster—more acute toxic effects expected, NPs < 50 nm are used by the RES—however liver and spleen have oxidative stress. |
Shape/Structure | Tissue toxicity is influenced by spherical or rod-like filament or plate-shaped structure | Non-spherical NPs are more exposed to blood flow and exhibit increased toxicity effects regarding hemolysis and/or platelet aggregation. |
Surface | Toxicity caused by different NP surfaces (altered absorption and/or interference with cell adhesion) | Cationic NPs could cause hemolysis and blood clotting, while anionic particles are non-toxic. Zwitterionic and neutral organic surface coatings can reduce penetration of nanoparticles through cellular membranes. |
Concentration | Toxicity caused by NP density (total particles/dose) | The risk for toxic side-effects rises with a higher concentration/density of NP. |
Formulation | Toxicity can arise from the NP carrier (incompatible organic or inorganic materials). | Composition of non-toxic materials/carriers should be prioritized. Whilst biodegradability can afford issues with stability in vivo, when using non-biodegradable systems, persistence should be monitored to ensure no long-term toxic effects are associated with this. The stable binding of therapeutic radionuclides to nanoparticles (including decay daughters) is essential. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleynhans, J.; Sathekge, M.; Ebenhan, T. Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy. Materials 2021, 14, 4784. https://doi.org/10.3390/ma14174784
Kleynhans J, Sathekge M, Ebenhan T. Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy. Materials. 2021; 14(17):4784. https://doi.org/10.3390/ma14174784
Chicago/Turabian StyleKleynhans, Janke, Mike Sathekge, and Thomas Ebenhan. 2021. "Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy" Materials 14, no. 17: 4784. https://doi.org/10.3390/ma14174784
APA StyleKleynhans, J., Sathekge, M., & Ebenhan, T. (2021). Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy. Materials, 14(17), 4784. https://doi.org/10.3390/ma14174784