Multiple Intermolecular Interaction to Improve the Abrasion Resistance and Wet Skid Resistance of Eucommia Ulmoides Gum/Styrene Butadiene Rubber Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EEUG
2.3. MTES Modified SiO2
2.4. Preparation of Rubber Composites
2.5. Testing and Characterization
2.5.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.5.2. X-ray Photoelectron Spectroscopy (XPS)
2.5.3. Determination of Bonding Rubber
2.5.4. Curing Characteristics
2.5.5. Mechanical Performance Testing
2.5.6. Akron Abrasion Test
2.5.7. SEM Analysis
2.5.8. Wet Sliding Friction Test
3. Results
3.1. FTIR Analysis of the Interaction between SiO2 and Enhancer
3.2. XPS Analysis of the Interaction between SiO2 and Enhancer
3.3. Microstructure of Rubber Composite
3.4. Binder Content of Rubber Composite
3.5. Curing Characteristics of Rubber Composites
3.6. Rubber Processing Analyzer of Rubber Composites
3.7. Mechanical Performance of Rubber Composites
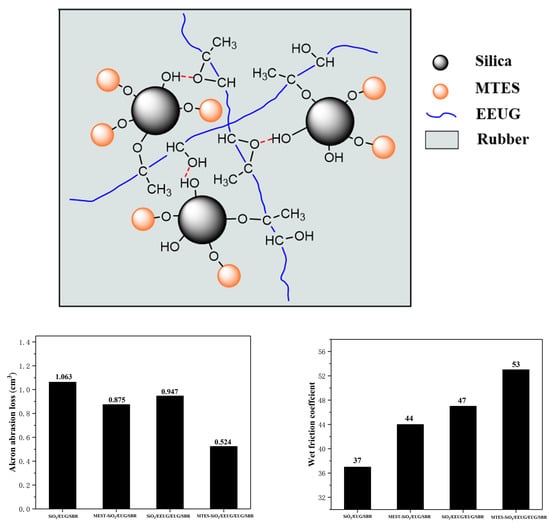
3.8. Wear Resistance and Wet Skid Resistance of Rubber Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Waddell, W.H.; Evans, L.R. Use of nonblack fillers in tire compounds. Rubber Chem. Technol. 1996, 69, 377–423. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of natural rubber with silica/carbon black hybrid filler. Polym. Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Tse, M.F. BIMS/filler interactions. I. Effects of filler structure. J. Appl. Polym. Sci. 2006, 100, 4943–4956. [Google Scholar] [CrossRef]
- Hilonga, A.; Kim, J.K.; Sarawade, P.B.; Quang, D.V.; Shao, G.N.; Elineema, G.; Kim, H.T. Synthesis of mesoporous silica with superior properties suitable for green tire. J. Ind. Eng. Chem. 2012, 18, 1841–1844. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Wen, S.; Lu, Y.; Yang, H.; Zhang, L.; Liu, L. Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos. Part. A Appl. Sci. Manuf. 2014, 62, 52–59. [Google Scholar] [CrossRef]
- Pan, X.D. Impact of reinforcing filler on the dynamic moduli of elastomer compounds under shear deformation in relation to wet sliding friction. Rheol. Acta 2005, 44, 379–395. [Google Scholar] [CrossRef]
- Gal, L.A.; Yang, X.; Klüppel, M. Evaluation of sliding friction and contact mechanics of elastomers based on dynamic-mechanical analysis. J. Chem. Phys. 2005, 123, 014704. [Google Scholar] [CrossRef]
- Gao, W.; Lu, J.; Song, W.; Hu, J.; Han, B. Interfacial interaction modes construction of various functional SSBR–silica towards high filler dispersion and excellent composites performances. RSC Adv. 2019, 9, 18888–18897. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.X.; Wu, Y.P.; Li, W.J.; Zhang, L.Q. Influence of filler type on wet skid resistance of SSBR/BR composites: Effects from roughness and micro-hardness of rubber surface. Appl. Surf. Sci. 2011, 257, 2058–2065. [Google Scholar] [CrossRef]
- Palraj, S.; Selvaraj, M.; Maruthan, K.; Rajagopal, G. Corrosion and wear resistance behavior of nano-silica epoxy composite coatings. Prog. Org. Coat. 2015, 81, 132–139. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X.; Li, S.D.; Chen, Y.; Huang, M.F. Self-assembled natural rubber/silica nanocomposites: Its preparation and characterization. Compos. Sci. Technol. 2007, 67, 3130–3139. [Google Scholar] [CrossRef]
- Mizutani, T.; Arai, K.; Miyamoto, M.; Kimura, Y. Application of silica-containing nano-composite emulsion to wall paint: A new environmentally safe paint of high performance. Prog. Org. Coat. 2006, 55, 276–283. [Google Scholar] [CrossRef]
- Doan, V.A.; Nobukawa, S.; Ohtsubo, S.; Tada, T.; Yamaguchi, M. Selective migration of silica particles between rubbers. J. Polym. Res. 2013, 20, 1–6. [Google Scholar] [CrossRef]
- Reuvekamp, L.A.; Ten Brinke, J.W.; Van Swaaij, P.J.; Noordermeer, J.W. Effects of time and temperature on the reaction of TESPT silane coupling agent during mixing with silica filler and tire rubber. Rubber Chem. Technol. 2002, 75, 187–198. [Google Scholar] [CrossRef]
- Qu, L.; Yu, G.; Wang, L.; Li, C.; Zhao, Q.; Li, J. Effect of filler–elastomer interactions on the mechanical and nonlinear viscoelastic behaviors of chemically modified silica-reinforced solution-polymerized styrene butadiene rubber. J. Polym. Sci. 2012, 126, 116–126. [Google Scholar] [CrossRef]
- Venter, S.A.S.; Kunita, M.H.; Matos, R.; Nery, R.C.; Radovanovic, E.; Muniz, E.C.; Girotto, E.M.; Rubira, A.F. Thermal and scanning electron microscopy/energy-dispersive spectroscopy analysis of styrene–butadiene rubber–butadiene rubber/silicon dioxide and styrene–butadiene rubber–butadiene rubber/carbon black–silicon dioxide composites. J. Appl. Polym. Sci. 2005, 96, 2273–2279. [Google Scholar] [CrossRef]
- Prasertsri, S.; Rattanasom, N. Mechanical and damping properties of silica/natural rubber composites prepared from latex system. Polym. Test. 2011, 30, 515–526. [Google Scholar] [CrossRef]
- Katueangngan, K.; Tulyapitak, T.; Saetung, A.; Soontaranon, S.; Nithi-uthai, N. Renewable interfacial modifier for silica filled natural rubber compound. Procedia Chem. 2016, 19, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Alex, R.; Nando, G.B. Cardanol grafted natural rubber: A green substitute to natural rubber for enhancing silica filler dispersion. J. Appl. Polym. Sci. 2016, 133, 43057. [Google Scholar] [CrossRef]
- Lopez, J.F.; Perez, L.D.; Lopez, B.L. Effect of silica modification on the chemical interactions in NBR-based composites. J. Appl. Polym. Sci. 2011, 122, 2130–2138. [Google Scholar] [CrossRef]
- Wang, M.; Morris, M.D.; Kutsovsky, Y. Effect of fumed silica surface area on silicone rubber reinforcement. KGK-Kaut. Gummi Kunst. 2008, 61, 107. [Google Scholar]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W. Optimization of mixing conditions for silica-reinforced natural rubber tire tread compounds. Rubber Chem. Technol. 2012, 85, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhao, S.; Zhang, X.; Li, X.; Bai, Y. Preparation, structure, and properties of solution-polymerized styrene-butadiene rubber with functionalized end-groups and its silica-filled composites. Polymer 2014, 55, 1964–1976. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, S.; Yang, Y.; Zhang, X.; Wu, Y. Structure and properties of star-shaped solution-polymerized styrene-butadiene rubber and its co-coagulated rubber filled with silica/carbon black-I: Morphological structure and mechanical properties. Polym. Advan. Technol. 2009, 20, 818–825. [Google Scholar] [CrossRef]
- Wang, M.J. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, Q.; Zhang, L.; Wang, Y.; Wu, Y. Tailoring silica-rubber interactions by interface modifiers with multiple functional groups. RSC Adv. 2017, 7, 38915–38922. [Google Scholar] [CrossRef] [Green Version]
- Hashim, A.S.; Azahari, B.; Ikeda, Y.; Kohjiya, S. The effect of bis (3-triethoxysilylpropyl) tetrasulfide on silica reinforcement of styrene-butadiene rubber. Rubber Chem. Technol. 1998, 71, 289–299. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.S.; Lim, S.H.; Jang, S.H.; Kim, D.H.; Park, N.H.; Jung, J.W.; Choi, J. The investigation of the silica-reinforced rubber polymers with the methoxy type silane coupling agents. Polymers 2020, 12, 3058. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Zhang, M.Q.; Rong, M.Z.; Friedrich, K. Silica nanoparticles filled polypropylene: Effects of particle surface treatment, matrix ductility and particle species on mechanical performance of the composites. Compos. Sci. Technol. 2005, 65, 635–645. [Google Scholar] [CrossRef]
- Kapgate, B.P.; Das, C.; Basu, D.; Das, A.; Heinrich, G. Rubber composites based on silane-treated stöber silica and nitrile rubber: Interaction of treated silica with rubber matrix. J. Elastom. Plast. 2015, 47, 248–261. [Google Scholar] [CrossRef]
- Bertora, A.; Castellano, M.; Marsano, E.; Alessi, M.; Conzatti, L.; Stagnaro, P.; Colucci, G.; Priola, A.; Turturro, A. A new modifier for silica in reinforcing SBR elastomers for the tyre industry. Macromol. Mater. Eng. 2011, 296, 455–464. [Google Scholar] [CrossRef]
- Dong, H.; Luo, Y.; Lin, J.; Bai, J.; Chen, Y.; Zhong, B.; Jia, D. Effects of modified silica on the co-vulcanization kinetics and mechanical performances of natural rubber/styrene–butadiene rubber blends. J. Appl. Polym. Sci. 2020, 137, 48838. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Liu, L.; Zhang, F.; Zhang, L.; Wen, S.; Lu, Y.; Yang, H.; Shen, J. Surface modification of silica by two-step method and properties of Solution Styrene Butadiene Rubber (SSBR) nanocomposites filled with modified silica. Compos. Sci. Technol. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Dierkes, W.K.; Noordermeer, J.W. Elucidation of filler-to-filler and filler-to-rubber interactions in silica-reinforced natural rubber by TEM Network Visualization. Eur. Polym. J. 2014, 54, 118–127. [Google Scholar] [CrossRef]
- Sengloyluan, K.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W. Silica-reinforced tire tread compounds compatibilized by using epoxidized natural rubber. Eur. Polym. J. 2014, 51, 69–79. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Luo, Y.; Jia, D.; Peng, Z. Interfacial interaction between the epoxidized natural rubber and silica in natural rubber/silica composites. Appl. Surf. Sci. 2015, 328, 306–313. [Google Scholar] [CrossRef]
- Xia, L.; Wang, Y.; Ma, Z.; Du, A.; Qiu, G.; Xin, Z. Preparation of epoxidized Eucommia ulmoides gum and its application in Styrene-Butadiene Rubber (SBR)/silica composites. Polym. Adv. Technol. 2017, 28, 94–101. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Xia, L.; Shen, M.; Xin, Z.; Kim, J. Role of epoxidized natural Eucommia ulmoides gum in modifying the interface of styrene-butadiene rubber/silica composites. Polym. Adv. Technol. 2019, 30, 2968–2976. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J. Surface free energies and steam stability of methyl-modified silica membranes. J. Porous. Mat. 2009, 16, 737. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Wang, X. Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vib. Spectrosc. 2008, 46, 1–7. [Google Scholar] [CrossRef]
- Xiong, W.; Yang, D.; Yang, R.; Li, Y.; Zhou, H.; Qiu, X. Preparation of lignin-based silica composite submicron particles from alkali lignin and sodium silicate in aqueous solution using a direct precipitation method. Ind. Crop. Prod. 2015, 74, 285–292. [Google Scholar] [CrossRef]
- Cao, F.; Kim, D.; Li, X.; Feng, C.; Song, Y. Synthesis of polyaluminocarbosilane and reaction mechanism study. J. Appl. Polym. Sci. 2002, 85, 2787–2792. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Wu, L.; Chen, Y.; Luo, Y.; Jia, D.; Peng, Z. Influence of acetone extract from natural rubber on the structure and interface interaction in NR/silica composites. Appl. Surf. Sci. 2017, 423, 43–52. [Google Scholar] [CrossRef]
- Xu, C.; Xu, G.; Du, M.; Zhu, H.; Fu, Y.; Zhang, X. Effects of plant polyphenols on the interface and mechanical properties of rubber/silica composites. Polym. Polym. Compos. 2012, 20, 853–860. [Google Scholar] [CrossRef]
- Sun, D.; Li, X.; Zhang, Y.; Li, Y. Effect of modified nano-silica on the reinforcement of styrene butadiene rubber composites. J. Macromol. Sci. Part B 2011, 50, 1810–1821. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Anyszka, R.; Morishita, Y.; Ronza, D.R.; Blume, A. Comparison between SBR compounds filled with in-situ and ex-situ silanized silica. Polymers 2021, 13, 281. [Google Scholar] [CrossRef]
- Sengloyluan, K.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W. Reduced ethanol emissions by a combination of epoxidized natural rubber and silane coupling agent for silica-reinforced natural rubber-based tire treads. Rubber Chem. Technol. 2016, 89, 419–435. [Google Scholar] [CrossRef]
- Choi, S.S. Influence of storage time and temperature and silane coupling agent on bound rubber formation in filled styrene–butadiene rubber compounds. Polym. Test. 2002, 21, 201–208. [Google Scholar] [CrossRef]
- Bach, Q.V.; Vu, C.M.; Vu, H.T. Effects of co-silanized silica on the mechanical properties and thermal characteristics of natural rubber/styrene-butadiene rubber blend. Silicon 2020, 12, 1799–1809. [Google Scholar] [CrossRef]
- Sarkawi, S.S.; Aziz, A.K.C.; Rahim, R.A.; Ghani, R.A.; Kamaruddin, A.N. Properties of epoxidized natural rubber tread compound: The hybrid reinforcing effect of silica and silane system. Polym. Polym. Compos. 2016, 24, 775–782. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, X.; Gong, G.; Zheng, J.; Liang, T.; Yin, C.; Zhang, Q. Influence of particle type and silane coupling agent on properties of particle-reinforced styrene-butadiene rubber. Polym-Plast. Technol. 2012, 51, 268–272. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, Q.; Gu, J.; Zhao, Z.; Zheng, J.; Gong, G.; Liang, T.; Zhang, H. Cure characteristics and mechanical properties of vinyltriethoxysilane grafted styrene-butadiene rubber/silica blends. Polym-Plast. Technol. 2012, 51, 1218–1222. [Google Scholar] [CrossRef]
- Manna, A.K.; De, P.P.; Tripathy, D.K.; De, S.K.; Peiffer, D.G. Bonding between precipitated silica and epoxidized natural rubber in the presence of silane coupling agent. J. Appl. Polym. Sci. 1999, 74, 389–398. [Google Scholar] [CrossRef]
- Mensah, B.; Agyei-Tuffour, B.; Nyankson, E.; Bensah, Y.D.; Dodoo-Arhin, D.; Bediako, J.K.; Onwona-Agyeman, B.; Yaya, A. Preparation and characterization of rubber blends for industrial tire tread fabrication. Int. J. Polym. Sci. 2018, 2018, 2473286. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- George, K.M.; Varkey, J.K.; Thomas, K.T.; Mathew, N.M. Epoxidized natural rubber as a reinforcement modifier for silica-filled nitrile rubber. J. Appl. Polym. Sci. 2002, 85, 292–306. [Google Scholar] [CrossRef]
- Sengloyluan, K.; Sahakaro, K.; Noordermeer, J.W. Silica-reinforced natural rubber compounds compatibilized through the use of epoxide functional groups and tespt combination. Adv. Mat. Res. 2013, 844, 272–275. [Google Scholar] [CrossRef]
- Singh, V.K.; Gope, P.C. Silica-styrene-butadiene rubber filled hybrid composites: Experimental characterization and modeling. J. Reinf. Plast. Comp. 2010, 29, 2450–2468. [Google Scholar] [CrossRef]
- Martin, P.J.; Brown, P.; Chapman, A.V.; Cook, S. Silica-reinforced epoxidized natural rubber tire treads-performance and durability. Rubber Chem. Technol. 2015, 88, 390–411. [Google Scholar] [CrossRef]
- Seo, G.; Park, S.M.; Ha, K.; Choi, K.T.; Hong, C.K.; Kaang, S. Effectively reinforcing roles of the networked silica prepared using 3, 3′-bis (triethoxysilylpropyl) tetrasulfide in the physical properties of SBR compounds. J. Mater. Sci. 2010, 45, 1897–1903. [Google Scholar] [CrossRef]












| Sample | #1 | #2 | #3 | #4 |
|---|---|---|---|---|
| SBR | 70 | 70 | 70 | 70 |
| EUG | 30 | 30 | 24 | 24 |
| EEUG | 0 | 0 | 6 | 6 |
| SiO2 | 30 | 0 | 30 | 0 |
| MTES-SiO2 | 0 | 30 | 0 | 30 |
| ZnO | 5 | 5 | 5 | 5 |
| SA | 4 | 4 | 4 | 4 |
| DM | 2 | 2 | 2 | 2 |
| Antioxidant 4020 | 1 | 1 | 1 | 1 |
| S | 2.5 | 2.5 | 2.5 | 2.5 |
| Sample | Element Constitution (%) | ||
|---|---|---|---|
| Si | C | O | |
| SiO2 | 26.91 | 18.85 | 54.24 |
| MTES-SiO2 | 24.73 | 26.51 | 48.75 |
| EEUG-SiO2 | 11.13 | 66.23 | 22.64 |
| EEUG-MTES-SiO2 | 6.05 | 82.41 | 11.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, K.; Xiong, Y. Multiple Intermolecular Interaction to Improve the Abrasion Resistance and Wet Skid Resistance of Eucommia Ulmoides Gum/Styrene Butadiene Rubber Composite. Materials 2021, 14, 5246. https://doi.org/10.3390/ma14185246
Li M, Wang K, Xiong Y. Multiple Intermolecular Interaction to Improve the Abrasion Resistance and Wet Skid Resistance of Eucommia Ulmoides Gum/Styrene Butadiene Rubber Composite. Materials. 2021; 14(18):5246. https://doi.org/10.3390/ma14185246
Chicago/Turabian StyleLi, Mingyang, Kuiye Wang, and Yuzhu Xiong. 2021. "Multiple Intermolecular Interaction to Improve the Abrasion Resistance and Wet Skid Resistance of Eucommia Ulmoides Gum/Styrene Butadiene Rubber Composite" Materials 14, no. 18: 5246. https://doi.org/10.3390/ma14185246
APA StyleLi, M., Wang, K., & Xiong, Y. (2021). Multiple Intermolecular Interaction to Improve the Abrasion Resistance and Wet Skid Resistance of Eucommia Ulmoides Gum/Styrene Butadiene Rubber Composite. Materials, 14(18), 5246. https://doi.org/10.3390/ma14185246