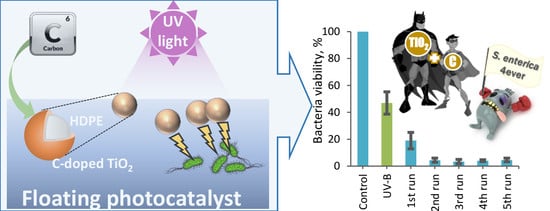
Photocatalytic Inactivation of Salmonella typhimurium by Floating Carbon-Doped TiO2 Photocatalyst
Abstract
:1. Introduction
2. Methodology
2.1. Synthesis
2.2. Structural Characterization of the Films
2.3. Bacteria Inactivation
2.3.1. Bacteria Cultivation
2.3.2. Bacteria Inactivation Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Letifi, H.; Dridi, D.; Litaiem, Y.; Ammar, S.; Dimassi, W.; Chtourou, R. High efficient and cost Effective titanium doped tin dioxide based photocatalysts synthesized via co-precipitation approach. Catalysts 2021, 11, 803. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Rajbongshi, B.M. Photocatalyst: Mechanism, challenges, and strategy for organic contaminant degradation. In Handbook of Smart Photocatalytic Materials; Mustansar Hussain, C., Mishra, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 127–149. ISBN 978-0-12-819049-4. [Google Scholar]
- Nasir, A.M.; Jaafar, J.; Aziz, F.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F.; Aziz, M. A review on floating nanocomposite photocatalyst: Fabrication and applications for wastewater treatment. J. Water Process Eng. 2020, 36, 101300. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrogen Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Mollavali, M.; Rohani, S.; Elahifard, M.; Behjatmanesh-Ardakani, R.; Nourany, M. Band gap reduction of (Mo+N) co-doped TiO2 nanotube arrays with a significant enhancement in visible light photo-conversion: A combination of experimental and theoretical study. Int. J. Hydrogen Energy 2021, 46, 21475–21498. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped titanium dioxide: An overview of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Changanaqui, K.; Brillas, E.; Alarcón, H.; Sirés, I. ZnO/TiO2/Ag2Se nanostructures as photoelectrocatalysts for the degradation of oxytetracycline in water. Electrochim. Acta 2020, 331, 135194. [Google Scholar] [CrossRef]
- Pishkar, N.; Jedi-soltanabadi, Z.; Ghoranneviss, M. Reduction in the band gap of anodic TiO2 nanotube arrays by H2 plasma treatment. Results Phys. 2018, 10, 466–468. [Google Scholar] [CrossRef]
- Gharaei, S.K.; Abbasnejad, M.; Maezono, R. Bandgap reduction of photocatalytic TiO2 nanotube by Cu doping. Sci. Rep. 2018, 8, 14192. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gong, H.; Zou, M.; Jiang, H.; Abolaji, R.S.; Tareen, A.K.; Hakala, B.V.; Yang, M. Mo-N-co-doped mesoporous TiO2 microspheres with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2017, 96, 10–17. [Google Scholar] [CrossRef]
- Peng, S.; Yang, Y.; Li, G.; Jiang, J.; Jin, K.; Yao, T.; Zhang, K.; Cao, X.; Wang, Y.; Xu, G. Effect of N2 flow rate on the properties of N doped TiO2 films deposited by DC coupled RF magnetron sputtering. J. Alloys Compd. 2016, 678, 355–359. [Google Scholar] [CrossRef]
- Guo, F.; Liu, J.; Zhang, W.; Yu, Z.; Liu, Y.; Liang, W. Synthesis of Cu,N-doped TiO2 nanotube by a novel magnetron sputtering method and its photoelectric property. Vacuum 2019, 165, 223–231. [Google Scholar] [CrossRef]
- Yang, J.; Hu, Y.; Jin, C.; Zhuge, L.; Wu, X. Structural and optical properties of Er-doped TiO2 thin films prepared by dual-frequency magnetron co-sputtering. Thin Solid Films 2017, 637, 9–13. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- De Vietro, N.; Tursi, A.; Beneduci, A. Photocatalytic inactivation of Escherichia coli bacteria in water using low pressure plasma deposited TiO2 cellulose fabric. Photochem. Photobiol. Sci. 2019, 18, 2248–2258. [Google Scholar] [CrossRef]
- Sboui, M.; Nsib, M.F.; Rayes, A.; Ochiai, T.; Houas, A. Application of solar light for photocatalytic degradation of Congo red by a floating salicylic acid-modified TiO2/palm trunk photocatalyst. Comptes Rendus Chim. 2017, 20, 181–189. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.X.; Bu, Y.; Zhang, J.; Wang, X.X.; Huang, J.; Chen, J.; Zhao, J. Preparation, characterization, and photocatalytic activity evaluation of Fe–N-codoped TiO2/fly ash cenospheres floating photocatalyst. Environ. Sci. Pollut. Res. 2016, 23, 22793–22802. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; Pang, J.; Qing, X.; Zhai, J.; Li, Q. Novel polypyrrole-sensitized hollow TiO2/fly ash cenospheres: Synthesis, characterization, and photocatalytic ability under visible light. Appl. Surf. Sci. 2012, 258, 9989–9996. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Wang, X.; Song, J.; Huang, J.; Louangsouphom, B.; Zhao, J. Floating photocatalysts based on loading Bi/N-doped TiO2 on expanded graphite C/C (EGC) composites for the visible light degradation of diesel. RSC Adv. 2015, 5, 71922–71931. [Google Scholar] [CrossRef]
- Długosz, M.; Waś, J.; Szczubiałka, K.; Nowakowska, M. TiO2-coated EP as a floating photocatalyst for water purification. J. Mater. Chem. A 2014, 2, 6931–6938. [Google Scholar] [CrossRef]
- Faramarzpour, M.; Vossoughi, M.; Borghei, M. Photocatalytic degradation of furfural by titania nanoparticles in a floating-bed photoreactor. Chem. Eng. J. 2009, 146, 79–85. [Google Scholar] [CrossRef]
- Valdez-Castillo, M.; Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic inactivation of airborne microorganisms in continuous flow using perlite-supported ZnO and TiO2. Chem. Eng. J. 2019, 374, 914–923. [Google Scholar] [CrossRef]
- Shavisi, Y.; Sharifnia, S.; Hosseini, S.N.; Khadivi, M.A. Application of TiO2/perlite photocatalysis for degradation of ammonia in wastewater. J. Ind. Eng. Chem. 2014, 20, 278–283. [Google Scholar] [CrossRef]
- Xue, H.; Jiang, Y.; Yuan, K.; Yang, T.; Hou, J.; Cao, C.; Feng, K.; Wang, X. Floating photocatalyst of B-N-TiO2/expanded perlite: A sol-gel synthesis with optimized mesoporous and high photocatalytic activity. Sci. Rep. 2016, 6, 29902. [Google Scholar] [CrossRef]
- Sboui, M.; Lachheb, H.; Bouattour, S.; Gruttadauria, M.; La Parola, V.; Liotta, L.F.; Boufi, S. TiO2/Ag2O immobilized on cellulose paper: A new floating system for enhanced photocatalytic and antibacterial activities. Environ. Res. 2021, 198, 111257. [Google Scholar] [CrossRef]
- Sboui, M.; Nsib, M.F.; Rayes, A.; Swaminathan, M.; Houas, A. TiO2–PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. J. Environ. Sci. (China) 2017, 60, 3–13. [Google Scholar] [CrossRef]
- Martín de Vidales, M.J.; Nieto-Márquez, A.; Morcuende, D.; Atanes, E.; Blaya, F.; Soriano, E.; Fernández-Martínez, F. 3D printed floating photocatalysts for wastewater treatment. Catal. Today 2019, 328, 157–163. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Mahalingam, H. Novel Floating Ag+-Doped TiO2/Polystyrene Photocatalysts for the Treatment of Dye Wastewater. Ind. Eng. Chem. Res. 2014, 53, 16332–16340. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black carbon-doped TiO2 films: Synthesis, characterization and photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 382, 111941. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Tuckute, S.; Lelis, M.; Milcius, D. Development of photocatalytically active TiO2 thin films on expanded polystyrene foam using magnetron sputtering. Vacuum 2017, 143, 28–35. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Daugelavicius, R.; Pranevicius, L.; Lelis, M.; Milcius, D. Floating TiO2 photocatalyst for efficient inactivation of E. coli and decomposition of methylene blue solution. Sci. Total Environ. 2020, 720, 137600. [Google Scholar] [CrossRef]
- Kuliesiene, N.; Sakalauskaite, S.; Tuckute, S.; Urbonavicius, M.; Varnagiris, S.; Daugelavicius, R.; Lelis, M. TiO2 application for the photocatalytical inactivation of S. enterica, E. coli and M. luteus bacteria mixtures. Environ. Clim. Technol. 2020, 24, 418–429. [Google Scholar] [CrossRef]
- Urbonavicius, M.; Varnagiris, S.; Sakalauskaite, S.; Demikyte, E.; Tuckute, S.; Lelis, M. Application of Floating TiO2 Photocatalyst for Methylene Blue Decomposition and Salmonella typhimurium Inactivation. Catalysts 2021, 11, 794. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, D. Room temperature growth of nanocrystalline anatase TiO2 thin films by dc magnetron sputtering. Phys. B Condens. Matter 2010, 405, 1258–1266. [Google Scholar] [CrossRef]
- Musil, J.; Heřman, D.; Šícha, J. Low-temperature sputtering of crystalline TiO2 films. J. Vac. Sci. Technol. A 2006, 24, 521–528. [Google Scholar] [CrossRef]
- Twu, M.J.; Chiou, A.H.; Hu, C.C.; Hsu, C.Y.; Kuo, C.G. Properties of TiO2 films deposited on flexible substrates using direct current magnetron sputtering and using high power impulse magnetron sputtering. Polym. Degrad. Stab. 2015, 117, 1–7. [Google Scholar] [CrossRef]
- Kuo, C.G.; Hsu, C.Y.; Wang, S.S.; Wen, D.C. Photocatalytic characteristics of TiO2 films deposited by magnetron sputtering on polycarbonate at room temperature. Appl. Surf. Sci. 2012, 258, 6952–6957. [Google Scholar] [CrossRef]
- Chodun, R.; Skowronski, L.; Okrasa, S.; Wicher, B.; Nowakowska-Langier, K.; Zdunek, K. Optical TiO2 layers deposited on polymer substrates by the Gas Injection Magnetron Sputtering technique. Appl. Surf. Sci. 2019, 466, 12–18. [Google Scholar] [CrossRef]
- Loukopoulos, S.; Toumazatou, A.; Sakellis, E.; Xenogiannopoulou, E.; Boukos, N.; Dimoulas, A.; Likodimos, V. Heterostructured CoOx–TiO2 Mesoporous/Photonic Crystal Bilayer Films for Enhanced Visible-Light Harvesting and Photocatalysis. Materials 2020, 13, 4305. [Google Scholar] [CrossRef] [PubMed]
- NIST. NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database 20; Version 4.1. Available online: https://srdata.nist.gov/xps/ (accessed on 24 August 2021).
- Akhavan, O. Lasting antibacterial activities of Ag-TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Abdi, Y.; Mohajerzadeh, S. Synthesis of titania/carbon nanotube heterojunction arrays for photoinactivation of E. coli in visible light irradiation. Carbon N. Y. 2009, 47, 3280–3287. [Google Scholar] [CrossRef]






| Substrate | Fabrication Technique | Photocatalyst | Medium/Light | Photocatalytic Performance | Ref. |
|---|---|---|---|---|---|
| Cellulose fabric | Magnetron sputtering | TiO2 | Escherichia coli/UV-LED | 100%/1 h | [17] |
| Pieces of palm trunk | Sol–gel | Salicylic acid-modified anatase TiO2 | Congo red dye/Sunlight | 98.2%/3.5 h | [18] |
| Fly ash cenospheres | Sol–gel | Fe–N-co-doped TiO2 | Rhodamine B/Visible light | 89%/4 h | [19] |
| Fly ash cenospheres | Chemical synthesis and calcination | Polypyrrole-sensitized TiO2 | Methylene blue/Visible light | 55%/9 h | [20] |
| Expanded graphite C/C composites | Sol–gel | Bismuth/nitrogen-co-doped TiO2 | Diesel/Visible light | 83.8%/5 h | [21] |
| Perlite | Direct precipitation | TiO2 | Phenol/UV-A | 45%/3 h | [22] |
| Perlite | Chemical synthesis and calcination | TiO2 nanoparticles | Furfural/UV-C | 95%/2 h | [23] |
| Perlite | Chemical synthesis and calcination | TiO2 | Bioaerosols/UV-C | 40%/2 h | [24] |
| Perlite | Chemical synthesis and calcination | TiO2 nanoparticles | Ammonia/UV-C | 68%/3 h | [25] |
| Perlite | Sol–gel | B–N-co-doped TiO2 | Rhodamine B/Visible light | 94%/3 h | [26] |
| Cellulose paper | Dipping and hydrothermal treatment | TiO2/Ag2O composite | Aniline/Visible light | 97%/6 h | [27] |
| Small pieces of cork | Sol–gel | TiO2–polyaniline composite | Methyl orange/Sunlight | 95.2%/3.5 h | [28] |
| Low-density polyethylene | 3D printing | TiO2 | Methylene blue/UV | 14%/2 h | [29] |
| Polystyrene | Strewing solvent casting | Ag+-doped TiO2 | Methylene blue/UV | 86%/5 h | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Demikyte, E.; Tuckute, S.; Lelis, M. Photocatalytic Inactivation of Salmonella typhimurium by Floating Carbon-Doped TiO2 Photocatalyst. Materials 2021, 14, 5681. https://doi.org/10.3390/ma14195681
Varnagiris S, Urbonavicius M, Sakalauskaite S, Demikyte E, Tuckute S, Lelis M. Photocatalytic Inactivation of Salmonella typhimurium by Floating Carbon-Doped TiO2 Photocatalyst. Materials. 2021; 14(19):5681. https://doi.org/10.3390/ma14195681
Chicago/Turabian StyleVarnagiris, Sarunas, Marius Urbonavicius, Sandra Sakalauskaite, Emilija Demikyte, Simona Tuckute, and Martynas Lelis. 2021. "Photocatalytic Inactivation of Salmonella typhimurium by Floating Carbon-Doped TiO2 Photocatalyst" Materials 14, no. 19: 5681. https://doi.org/10.3390/ma14195681
APA StyleVarnagiris, S., Urbonavicius, M., Sakalauskaite, S., Demikyte, E., Tuckute, S., & Lelis, M. (2021). Photocatalytic Inactivation of Salmonella typhimurium by Floating Carbon-Doped TiO2 Photocatalyst. Materials, 14(19), 5681. https://doi.org/10.3390/ma14195681