Novel Liquid Chitosan-Based Biocoagulant for Treatment Optimization of Fish Processing Wastewater from a Moroccan Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Wastewater and Shrimp Exoskeleton Samples
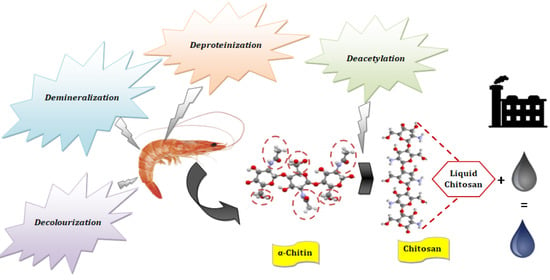
2.2. Preparation of the Natural Coagulant
2.2.1. Obtaining Chitin
2.2.2. Obtaining Chitosan
2.3. X-ray Diffraction (XRD)
2.4. Fourier Transforms Infrared Spectroscopy (FTIR)
2.5. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy (SEM/EDX)
2.6. Jar-Test
2.7. Analytical Methods
3. Results and Discussion
3.1. Shrimp Shell Characterization
3.1.1. Analysis by Scanning Electron Microscope
3.1.2. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy (SEM/EDX)
3.1.3. Analysis by Infrared Spectroscopy
3.1.4. Analysis by XRD Spectroscopy
3.2. Effect of Coagulant Dosage
3.3. Effect of pH
3.4. Optimization Using a Box–Behnken Experimental Design
3.4.1. Statistical Analysis
3.4.2. Modeling Approach of the Response Surface to Optimize the Variables Studied
3.4.3. Response Surface Plotting and Optimization of Turbidity Removal
3.4.4. Response Surface Plotting and Optimization of BOD5 Removal
3.5. Comparison with Other Coagulants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doukkali, M.R.; Kamili, A. Moroccan Fishery Production System and Its Dependence on the Rest of the World; OCP Policy Center: Rabat, Morocco, 2018; p. 13. [Google Scholar]
- Sousa, S.C.; Vázquez, J.A.; Pérez-Martín, R.I.; Carvalho, A.P.; Gomes, A.M. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules 2017, 22, 1545. [Google Scholar] [CrossRef] [Green Version]
- Coppola, D.; Lauritano, C.; Esposito, F.P.; Riccio, G.; Rizzo, C.; de Pescale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Elwakeel, K.Z. Environmental Application of Chitosan Resins for the Treatment of Water and Wastewater: A Review. J. Dispers. Sci. Technol. 2010, 31, 37–41. [Google Scholar]
- Roussy, J.; Van Vooren, M.; Dempsey, B.A.; Guibal, E. Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res. 2005, 39, 3247–3258. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Kassaveti, A. Original article Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Application of Chitosan for Treatment of Wastewaters. Rev. Environ. Contam. Toxicol. 2000, 163, 1–28. [Google Scholar]
- Devi, M.G.; Jessica, J.; Feroz, S. Dairy Wastewater Treatment Using Low Molecular Weight Crab Shell Chitosan. J. Inst. Eng. India Ser. 2013, 93, 9–14. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Bagasse wastewater treatment using biopolymer—A novel approach. J. Serb. Chem. Soc. 2014, 79, 897–909. [Google Scholar] [CrossRef]
- Venkatachalam, S. Treatment of egg processing industry effluent using chitosan as an adsorbent. J. Serb. Chem. Soc. 2014, 79, 743–757. [Google Scholar]
- Divakaran, R.; Pillai, V.N.S. Flocculation of river silt using chitosan. Water Res. 2002, 36, 2414–2418. [Google Scholar] [CrossRef]
- Bina, B.; Ebrahimi, A.; Hesami, F. The effectiveness of chitosan as coagulant aid in turbidity removal from water. Int. J. Environ. Health Eng. 2014, 2, 46–51. [Google Scholar] [CrossRef]
- Rodger, B.B.; Andrew, D.E.; Eugene, W.R. Standard Methods for the Examination of Water and Wastewater, 23th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association, Water Environment Federation: Denver, CO, USA, 2017; pp. 1–105. [Google Scholar]
- Paul, W.; Pillai CK, S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar]
- Zhanyong, G.; Ronge, X.; Song, L.; Zhimei, Z.; Xia, J.; Lin, W.P.L. The influence of molecular weight of quaternized chitosan on antifungal. Carbohydr. Polym. 2008, 71, 694–697. [Google Scholar]
- Saito, Y.; Putaux, J.L.; Okano, T.; Gaill, F.; Chanzy, H. Structural aspects of the swelling of β chitin in HCl and its conversion into α chitin. Macromolecules 1997, 30, 3867–3873. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. Chitin and chitosan fibres: A review. Bull. Mater. Sci. 1999, 22, 905–915. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Carboxymethylated chitins and chitosans. Carbohydr. Polym. 1988, 8, 1–21. [Google Scholar] [CrossRef]
- Onsosyen, E.; Skaugrud, O. Metal recovery using chitosan. J. Chem. Technol. Biotechnol. 1990, 49, 395–404. [Google Scholar] [CrossRef]
- Louhıchı, G.; Bousselmı, L.; Ghrabı, A.; Khounı, I. Process optimization via response surface methodology in the physico-chemical treatment of vegetable oil refinery wastewater. Environ. Sci. Pollut. Res. 2019, 26, 18993–19011. [Google Scholar] [CrossRef] [PubMed]
- Khouni, I.; Marrot, B.; Ben Amar, R. Decolourization of the reconstituted dye bath effluent by commercial laccase treatment: Optimization through response surface methodology. Chem. Eng. J. 2010, 156, 121–133. [Google Scholar] [CrossRef]
- Benyounis, K.Y.; Olabi, A.G.; Hashmi, M.S.J. Optimizing the laser-welded butt joints of medium carbon steel using RSM. J. Mater. Process. Technol. 2005, 164, 986–989. [Google Scholar] [CrossRef] [Green Version]
- MEMEE. Preservation of the Quality of Water Resources and Fight against Pollution, Discharge Limit Values to Be Respected by Spills (Pollution Standards); Minister Delegate to the Minister of Energy, Mines, Water and Environment in charge of the Environment: Rabat, Morocco, 2014; p. 13. [Google Scholar]
- Abidar, F.; Morghi, M.; Abali, M.; Sinan, F.; Chiban, M.; Eddaoudi, H.; Zebret, M. Orthophosphate ion adsorption on to raw shrimp shells. J. Water Sci. 2016, 29, 197–211. [Google Scholar]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nge, T.T.; Takemura, A.; Hori, N.; Ono, H. Characterization of uniaxially aligned chitin film by 2D FT-IR spectroscopy. Biomacromolecules 2005, 6, 1941–1947. [Google Scholar] [CrossRef]
- Włochowicz, A.; Szosland, L.; Biniaś, D.; Szumilewicz, J. Crystalline structure and mechanical properties of wet-spun dibutyrylchitin fibers and products of their alkaline treatment. J. Appl. Polym. Sci. 2004, 94, 1861–1868. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. The structure of α-chitin. J. Mol. Biol. 1978, 120, 167–181. [Google Scholar] [CrossRef]
- Ogawa, K.; Yui, T.; Okuyama, K. Three D structures of chitosan. Int. J. Biol. Macromol. 2004, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yui, T.; Imada, K.; Okuyama, K.; Obata, Y.; Suzuki, K.; Ogawa, K. Molecular and Crystal Structure of the Anhydrous Form of Chitosan. Macromolecules 1994, 27, 7601–7605. [Google Scholar]
- Yui, T.; Taki, N.; Sugiyama, J.; Hayashi, S. Exhaustive crystal structure search and crystal modeling of β-chitin. Int. J. Biol. Macromol. 2007, 40, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and Characterization of Chitin and Chitosan from Parapenaeus longirostris from Moroccan Local Sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Kim, S.-K. Chitin, Chitosan, Oligosaccharides and Their Derivatives Biological; Taylor & Francis Group: Boca Raton, FL, USA, 1992; Volume 66, pp. 1360–1364. [Google Scholar]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Assaad, E. Study of the Coagulation-Flocculation Process of the Montmorillonite-Chitosan System in the Elimination of Transition Metals; University of Quebec in Montreal: Montreal, QC, Canada, 2006; pp. 1–156. [Google Scholar]
- Arbia, W.; Adour, L.; Amrane, A.; Lounici, H. Optimization of medium composition for enhanced chitin extraction from Parapenaeus longirostris by Lactobacillus helveticus using response surface methodology. Food Hydrocoll. 2013, 31, 392–403. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Gu, L.; Guo, X.; Zhou, J. The zircon SHRIMP chronology and trace element geochemistry of the Carboniferous volcanic rocks in western Tianshan Mountains. Chin. Sci. Bull. 2005, 50, 2201–2212. [Google Scholar] [CrossRef]
- Di Mario, F.; Rapanà, P.; Tomati, U.; Galli, E. Chitin and chitosan from Basidiomycetes. Int. J. Biol. Macromol. 2008, 43, 8–12. [Google Scholar] [CrossRef]
- Arbia, W.; Arbua, L.; Adour, L.; Amrane, A.; Benhadji, A.; Lounci, H. Characterization by spectrometric methods of chitin produced from white shrimp shells of Parapenaeus longirostris by Lactobacillus helveticus cultivated on glucose or date waste. Alger. J. Envion. Sci. Technol. 2019, 5, 955–963. [Google Scholar]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.F. Analysis of Chitin and Chitosan. Chitin Chem. 1992, 130, 1–462. [Google Scholar]
- Basosidik, S.; Pankaew, P.; Hoonnivathana, E.; Limsuwan, P.; Naemchanthara, K. Characterization of chitin extracted from waste sources via XRD, FTIR, and TGA techniques. Adv. Mater. Res. 2012, 488, 1404–1408. [Google Scholar] [CrossRef]
- Nouj, N.; Heddadi, N.; Azougarh, Y.; Hafid, N.; El Alem, N. A combined treatment of municipal solid waste landfill leachate using Opuntia ficus-indica cactus as coagulant and titaniferous sand as filter material. Nanotechnol. Environ. Eng. 2021, 6, 14. [Google Scholar] [CrossRef]
- Chethana, M.; Gayatri, S.L.; Vinay, B.M.; Vivek, R.V.; Raja, S. Application of biocoagulant Acanthocereus tetragonus (Triangle cactus) in dye wastewater treatment. J. Environ. Res. Dev. 2015, 9, 813–821. [Google Scholar]
- Patel, H.; Vashi, R.T. Comparison of naturally prepared coagulants for removal of COD and color from textile wastewater. Glob. NEST J. 2013, 15, 522–528. [Google Scholar]
- Sila, A.; Mlaik, N.; Sayari, N.; Balti, R.; Bougatef, A. Chitin and Chitosan Extracted from Shrimp Waste Using Fish Proteases Aided Process: Efficiency of Chitosan in the Treatment of Unhairing Effluents. J. Polym. Environ. 2014, 22, 78–87. [Google Scholar] [CrossRef]
- Rizzo, L.; Lofrano, G.; Grassi, M.; Belgiorno, V. Pre-treatment of olive mill wastewater by chitosan coagulation and advanced oxidation processes. Sep. Purif. Technol. 2008, 63, 648–653. [Google Scholar] [CrossRef]
- Park, T.; Ampunan, V.; Maeng, S.; Chung, E. Application of steel slag coated with sodium hydroxide to enhance precipitation-coagulation for phosphorus removal. Chemosphere 2017, 167, 91–97. [Google Scholar] [CrossRef]
- Guerrero, L.; Omil, F.; Méndez, R.; Lema, J.M. Protein recovery during the overall treatment of wastewaters from fish-meal factories. Bioresour. Technol. 1998, 63, 221–229. [Google Scholar] [CrossRef]
- García, M.A. Treatment of Wastewater from Fish Processing Industry using Chitosan Acid Salts. Int. J. Water Wastewater Treat. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Genovese, C.V.; González, J.F. Solids removal by coagulation from fisheries waste waters. Water SA 1998, 24, 371–372. [Google Scholar]
- Chung, Y.C. Improvement of aquaculture wastewater using chitosan of different degrees of deacetylation. Environ. Technol. 2006, 27, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Selmer-Olsen, E.; Ratnaweera, H.C.; Pehrson, R. A novel treatment process for dairy wastewater with chitosan produced shrimp-shell waste. Water Sci. Technol. 1996, 34, 33–40. [Google Scholar] [CrossRef]
- Tak, B.-Y.; Tak, B.-S.; Kim, Y.-J.; Park, Y.-J.; Yoon, Y.-H.; Min, G.-H. Optimization of color and COD removal from livestock wastewater by electrocoagulation process: Application of Box-Behnken design (BBD). J. Ind. Eng. Chem. 2015, 28, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Elmoubarki, R.; Taoufik, M.; Moufti, A.; Tounsadi, H.; Mahjoubi, F.Z.; Bouabi, Y.; Qourzal, S.; Abdennouri, M.; Barka, N. Box-Behnken experimental design for the optimization of methylene blue adsorption onto Aleppo pine cones. J. Mater. Environ. Sci. 2017, 8, 2184–2191. [Google Scholar]
- Cojocaru, C.; Pascariu Dorneanu, P.; Airinei, A.; Olaru, N.; Samoila, P.; Rotaru, A. Design and evaluation of electrospun polysulfone fibers and polysulfone/NiFe2O4 nanostructured composite as sorbents for oil spill cleanup. J. Taiwan Inst. Chem. Eng. 2017, 70, 267–281. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; do Carmo Nascimento, I.O.; Matos Queiroz, L.; Yuhyi, M.; Tadza, M.; Picos-Corrales, L.A.; Pei, H.; et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef] [Green Version]
- Ruhsing Pan, J.; Huang, C.; Chen, S.; Chung, Y.C. Evaluation of a modified chitosan biopolymer for coagulation of colloidal particles. Colloids Surf. A Physicochem. Eng. Asp. 1999, 147, 359–364. [Google Scholar] [CrossRef]















| Coded Variables | Parameters | Coded Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | coagulant concentration (mg/0.5 L) | 3 | 6 | 9 |
| X2 | initial pH | 8 | 10 | 12 |
| X3 | temperature (°C) | 16 | 20 | 24 |
| Run No. | Experimental Parameters | Responses | |||
|---|---|---|---|---|---|
| X1 Coagulant Dose (mL) | X2 Initial pH | X3 Temperature (°C) | Y1 Turbidity Removal (%) | Y2 BOD5 Removal (%) | |
| 1 | 3.00 | 8.00 | 20.00 | 93.40 | 39.52 |
| 2 | 9.00 | 8.00 | 20.00 | 89.10 | 26.74 |
| 3 | 3.00 | 12.00 | 20.00 | 95.90 | 50.23 |
| 4 | 9.00 | 12.00 | 20.00 | 94.66 | 35.40 |
| 5 | 3.00 | 10.00 | 16.00 | 92.97 | 31.21 |
| 6 | 9.00 | 10.00 | 16.00 | 86.88 | 18.39 |
| 7 | 3.00 | 10.00 | 24.00 | 94.54 | 39.33 |
| 8 | 9.00 | 10.00 | 24.00 | 93.11 | 20.90 |
| 9 | 6.00 | 8.00 | 16.00 | 91.54 | 26.10 |
| 10 | 6.00 | 12.00 | 16.00 | 95.42 | 47.76 |
| 11 | 6.00 | 8.00 | 24.00 | 97.90 | 40.53 |
| 12 | 6.00 | 12.00 | 24.00 | 97.81 | 46.86 |
| 13 | 6.00 | 10.00 | 20.00 | 98.30 | 53.39 |
| 14 | 6.00 | 10.00 | 20.00 | 98.32 | 53.50 |
| 15 | 6.00 | 10.00 | 20.00 | 98.31 | 53.61 |
| Parameter | Value | Moroccan Standard Limits for Direct Discharge into Effluents [24] | ||
|---|---|---|---|---|
| Min | Mean | Max | ||
| temperature | 12 | 18.5 | 25 | 30 °C |
| pH | 6.62 | 7.06 | 7.50 | 5.5–9.5 |
| conductivity | 9000 | 15,500 | 22,000 | 2700 µS/cm |
| turbidity | 850 | 925 | >1000 | - |
| BOD5 | 1700 | 2345.5 | 2990.9 | 100 mg O2/L |
| Parameter | Standard Deviation | R2 | R2 Adjusted | R2 Predicted | ||||||
| Turbidity | 0.759 | 0.983 | 0.953 | 0.730 | ||||||
| BOD5 | 1.549 | 0.994 | 0.984 | 0.906 | ||||||
| Coefficient | Value | F. Inflation | Standard Deviation | T Exp | Significance (%) | |||||
| Turbidity | BOD5 | Turbidity | BOD5 | Turbidity | BOD5 | Turbidity | BOD5 | Turbidity | BOD5 | |
| b0 | 98.32 | 53.50 | - | - | 0.44 | 0.90 | 224.31 | 59.83 | <0.01 *** | <0.01 *** |
| b1 | −1.63 | −7.36 | 1.00 | 1.00 | 0.27 | 0.55 | −6.08 | −13.44 | 0.174 ** | <0.01 *** |
| b2 | 1.48 | 5.92 | 1.00 | 1.00 | 0.27 | 0.55 | 5.52 | 10.81 | 0.268 ** | 0.0118 *** |
| b3 | 2.06 | 3.02 | 1.00 | 1.00 | 0.27 | 0.55 | 7.71 | 5.51 | 0.0587 *** | 0.268 ** |
| b1-1 | −4.42 | −14.19 | 1.01 | 1.01 | 0.40 | 0.81 | −11.20 | −17.61 | <0.01 *** | <0.01 *** |
| b2-2 | −0.63 | −1.34 | 1.01 | 1.01 | 0.40 | 0.81 | −1.60 | −1.66 | 17.1 | 15.8 |
| b3-3 | −2.02 | −11.85 | 1.01 | 1.01 | 0.40 | 0.81 | −5.12 | −14.70 | 0.372 ** | <0.01 *** |
| b1-2 | 0.77 | −0.51 | 1.00 | 1.00 | 0.38 | 0.77 | 2.02 | −0.66 | 10.0 | 53.7 |
| b1-3 | 1.17 | −1.40 | 1.00 | 1.00 | 0.38 | 0.77 | 3.07 | −1.81 | 2.78 * | 13.0 |
| b2-3 | −0.99 | −3.83 | 1.00 | 1.00 | 0.38 | 0.77 | −2.61 | −4.95 | 4.74 * | 0.429 ** |
| Sources of Variation | Sum of Squares | Degree of Freedom | Mean Square | Ratio | Significance (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Turbidity | BOD5 Removal | Turbidity | BOD5 Removal | Turbidity | BOD5 Removal | Turbidity | BOD5 Removal | Turbidity | BOD5 Removal | |
| regression | 167.68 | 2.02869 × 103 | 9 | 9 | 18.63 | 2.25410 × 102 | 32.32 | 93.95 | 0.07 *** | <0.01 *** |
| residual | 2.88 | 1.19955 × 101 | 5 | 5 | 0.58 | 2.39910 | ||||
| total | 170.56 | 2.04069 × 103 | 14 | 14 | ||||||
| X1 Coagulant Concentration (mg/0.5 L) Coded Value Real Value | X2 Initial pH Coded Value Real Value | X3 Temperature (°C) Coded Value Real Value | Y1 Turbidity Removal Rate (%) | Y2 BOD5 Removal Rate (%) |
|---|---|---|---|---|
| −0.23982 | 0.22863 | −0.20584 | 98.488 | 54.783 |
| 5.2805 | 10.457 | 19.177 |
| Parameter | Value before Treatment | Value after Treatment | Moroccan Standard Limit of Direct Discharge [24] | ||
|---|---|---|---|---|---|
| Liquid Chitosan | Ferric Chloride | Commercial Chitosan | |||
| turbidity | >1000 | 10.7 | 15.6 | 13.8 | - |
| BOD5 | 2990.9 | 201 | 412.5 | 209 | 100 mg O2/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouj, N.; Hafid, N.; El Alem, N.; Cretescu, I. Novel Liquid Chitosan-Based Biocoagulant for Treatment Optimization of Fish Processing Wastewater from a Moroccan Plant. Materials 2021, 14, 7133. https://doi.org/10.3390/ma14237133
Nouj N, Hafid N, El Alem N, Cretescu I. Novel Liquid Chitosan-Based Biocoagulant for Treatment Optimization of Fish Processing Wastewater from a Moroccan Plant. Materials. 2021; 14(23):7133. https://doi.org/10.3390/ma14237133
Chicago/Turabian StyleNouj, Nisrine, Naima Hafid, Noureddine El Alem, and Igor Cretescu. 2021. "Novel Liquid Chitosan-Based Biocoagulant for Treatment Optimization of Fish Processing Wastewater from a Moroccan Plant" Materials 14, no. 23: 7133. https://doi.org/10.3390/ma14237133
APA StyleNouj, N., Hafid, N., El Alem, N., & Cretescu, I. (2021). Novel Liquid Chitosan-Based Biocoagulant for Treatment Optimization of Fish Processing Wastewater from a Moroccan Plant. Materials, 14(23), 7133. https://doi.org/10.3390/ma14237133