Spherical Silica Modified with Magnesium and Ruthenium—Synthesis, Characterization and Catalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Cetyltrimethylammonium bromide (CTAB), >98% (Aldrich, St. Louis, MO, USA);
- Tetraethoxysilane (TEOS), >99% (Aldrich, St. Louis, MO, USA);
- Na2CO3, 99.5% (Aldrich, St. Louis, MO, USA);
- Ethanol, 96% (Stanlab, Lublin, Poland);
- Magnesium acetate tetrahydrate, >99% (Aldrich, St. Louis, MO, USA);
- Ruthenium trichloride hydrate, 35%–40% of Ru (Acros Organics, Geel, Belgium);
- 2-propanol (Stanlab, Lublin, Poland);
- Levulinic acid, >99% (Aldrich, St. Louis, MO, USA).
2.2. Synthesis of the Support
2.3. Modification with Magnesium
2.4. Further Modification with Silica
2.5. Modification with Ruthenium
2.6. Characterization
2.7. 2-Propanol Decomposition
2.8. Hydrogenation of Levulinic Acid
3. Results and Discussion
3.1. Morphology and Textural Properties of the Solids
3.2. States of the Metals
3.3. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.S.; Lu, G.Q.; Millar, G.J.; Li, X.S. Synthesis and characterization of highly ordered MCM-41 in an alkali-free system and its catalytic activity. Catal. Lett. 1996, 38, 33–37. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Winkel, P.; Lukens, W.W.; Zhao, D.; Yang, P.; Chmelka, B.F.; Stucky, G.D. Mesocellular Siliceous Foams with Uniformly Sized Cells and Windows. J. Am. Chem. Soc. 1998, 121, 254–255. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef]
- Du, X.; He, J. Spherical silica micro/nanomaterials with hierarchical structures: Synthesis and applications. Nanoscale 2011, 3, 3984–4002. [Google Scholar] [CrossRef] [PubMed]
- Gierszal, K.P.; Jaroniec, M. Carbons with Extremely Large Volume of Uniform Mesopores Synthesized by Carbonization of Phenolic Resin Film Formed on Colloidal Silica Template. J. Am. Chem. Soc. 2006, 128, 10026–10027. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Ma, Z.; Li, Y.; Shi, J. Synthesis of Core−Shell Structured Dual-Mesoporous Silica Spheres with Tunable Pore Size and Controllable Shell Thickness. J. Am. Chem. Soc. 2010, 132, 15144–15147. [Google Scholar] [CrossRef]
- Li, P.; Cao, C.-Y.; Chen, Z.; Liu, H.; Yu, Y.; Song, W.-G. Core–shell structured mesoporous silica as acid–base bifunctional catalyst with designated diffusion path for cascade reaction sequences. Chem. Commun. 2012, 48, 10541–10543. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Wang, P.; Werth, C.J.; Strathmann, T.J. Palladium Nanoparticles Encapsulated in Core–Shell Silica: A Structured Hydrogenation Catalyst with Enhanced Activity for Reduction of Oxyanion Water Pollutants. ACS Catal. 2014, 4, 3551–3559. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 14, 688–694. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.; Tsang, D.C.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-phase catalytic transfer hydrogenation and cyclization of levulinic acid and its esters to γ-valerolactone over metal oxide catalysts. Chem. Commun. 2011, 47, 12233–12235. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.R.; Kantam, M.L. Selective hydrogenation of levulinic acid into γ-valerolactone over Cu/Ni hydrotalcite-derived catalyst. Catal. Today 2018, 309, 189–194. [Google Scholar] [CrossRef]
- Wright, W.R.H.; Palkovits, R. Development of Heterogeneous Catalysts for the Conversion of Levulinic Acid to γ-Valerolactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef]
- Yan, L.; Yao, Q.; Fu, Y. Conversion of levulinic acid and alkyl levulinates into biofuels and high-value chemicals. Green Chem. 2017, 19, 5527–5547. [Google Scholar] [CrossRef]
- Yan, Z.-P.; Lin, L.; Liu, S. Synthesis of γ-Valerolactone by Hydrogenation of Biomass-derived Levulinic Acid over Ru/C Catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, M.; Kim, S.K.; Choi, Y.N.; Jung, M.; Oh, H.; Suh, Y.-W. Remarkably fast low-temperature hydrogen storage into aromatic benzyltoluenes over MgO-supported Ru nanoparticles with homolytic and heterolytic H2 adsorption. Appl. Catal. B Environ. 2021, 286, 119889. [Google Scholar] [CrossRef]
- Yao, Q.; Shi, W.; Feng, G.; Lu, Z.-H.; Zhang, X.-L.; Tao, D.-J.; Kong, D.; Chen, X. Ultrafine Ru nanoparticles embedded in SiO2 nanospheres: Highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane. J. Power Sources 2014, 257, 293–299. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Drozdek, M.; Rokicińska, A.; Natkański, P.; Michalik, M.; Kuśtrowski, P. Novel CuO-containing catalysts based on ZrO2 hollow spheres for total oxidation of toluene. Microporous Mesoporous Mater. 2019, 279, 446–455. [Google Scholar] [CrossRef]
- Fang, X.; Chen, C.; Liu, Z.; Liu, P.; Zheng, N. A cationic surfactant assisted selective etching strategy to hollow mesoporous silica spheres. Nanoscale 2011, 3, 1632–1639. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Abbott, D.F.; Mukerjee, S.; Petrykin, V.; Bastl, Z.; Halck, N.B.; Rossmeisl, J.; Krtil, P. Oxygen reduction on nanocrystalline ruthenia—Local structure effects. RSC Adv. 2014, 5, 1235–1243. [Google Scholar] [CrossRef]
- Morgan, D.J. Resolving ruthenium: XPS studies of common ruthenium materials. Surf. Interface Anal. 2015, 47, 1072–1079. [Google Scholar] [CrossRef]
- McCoy, A.; Bogan, J.; Brady, A.; Hughes, G. Oxidation of ruthenium thin films using atomic oxygen. Thin Solid Films 2015, 597, 112–116. [Google Scholar] [CrossRef]
- Bavand, R.; Yelon, A.; Sacher, E. X-ray photoelectron spectroscopic and morphologic studies of Ru nanoparticles deposited onto highly oriented pyrolytic graphite. Appl. Surf. Sci. 2015, 355, 279–289. [Google Scholar] [CrossRef]
- Garbassi, F.; Balducci, L.; Chiurlo, P.; Deiana, L. A study of surface modification of silica using XPS, DRIFT and NMR. Appl. Surf. Sci. 1995, 84, 145–151. [Google Scholar] [CrossRef]
- Ernst, M.A.; Sloof, W.G. Unraveling the oxidation of Ru using XPS. Surf. Interface Anal. 2008, 40, 334–337. [Google Scholar] [CrossRef]
- Hattori, H. Solid base catalysts: Fundamentals and their applications in organic reactions. Appl. Catal. A Gen. 2015, 504, 103–109. [Google Scholar] [CrossRef]
- Díez, V.K.; Apesteguía, C.R.; Di Cosimo, J.I. Acid–base properties and active site requirements for elimination reactions on alkali-promoted MgO catalysts. Catal. Today 2000, 63, 53–62. [Google Scholar] [CrossRef]
- Lahousse, C.; Bachelier, J.; Lavalley, J.-C.; Lauron-Pernot, H.; Le Govic, A.-M. Validity of using isopropanol decomposition as a test-reaction for the characterization of metal oxides basicity; comparison with results obtained from methylbutynol decomposition. J. Mol. Catal. 1994, 87, 329–332. [Google Scholar] [CrossRef]






| BET, m²·g−1 | Pore Diameter, nm | Pore Volume, cm³·g−1 | |
|---|---|---|---|
| NS | 505 | 3.3 | 0.43 |
| Ru/NS | 408 | 3.3 | 0.39 |
| Ru/4Mg/NS | 277 | 3.3 | 0.21 |
| 3Mg/NS | 428 | 3.1 | 0.32 |
| NS/3Mg/NS | 610 | 3.6 | 0.45 |
| Ru/NS/3Mg/NS | 478 | 3.1 | 0.31 |
| 5Mg/NS | 464 | 2.7 | 0.29 |
| NS/5Mg/NS | 494 | 3.3 | 0.38 |
| Ru/NS/5Mg/NS | 223 | 3.3 | 0.17 |
| 6Mg/NS | 321 | 2.6 | 0.19 |
| NS/6Mg/NS | 287 | 3.3 | 0.24 |
| Ru/NS/6Mg/NS | 196 | 3.3 | 0.15 |
| Ru, wt.% | RuO2 Diameter, nm 1 | Mg, wt.% | |||
|---|---|---|---|---|---|
| ICP | XPS | ICP | XPS | ||
| Ru/NS | 0.26 | 2.2 | 16.7 | - | - |
| Ru/4Mg/NS | 0.35 | 2.9 | 28.1 | 3.6 | 3.3 |
| Ru/NS/3Mg/NS | 0.32 | 3.0 | 20.6 | 2.5 | 1.5 |
| Ru/NS/5Mg/NS | 0.35 | 3.0 | 26.9 | 4.9 | 3.0 |
| Ru/NS/6Mg/NS | 0.38 | 3.1 | 23.8 | 5.9 | 4.9 |
| BE, eV | Ru/NS/3Mg/NS | Ru/NS/5Mg/NS | Ru/NS/6Mg/NS | Ru/NS | Ru/4Mg/NS |
|---|---|---|---|---|---|
| Ru 3p3/2 | 462.2 (50%) | 461.8 (46%) | 461.5 (53%) | 462.0 (41%) | 461.5 (53%) |
| 464.7 (50%) | 464.1 (54%) | 464.0 (47%) | 464.4 (59%) | 463.8 (60%) | |
| Ru 3d5/2 | 281.0 (78%) | 280.5 (40%) | 280.4 (47%) | 280.7 (64%) | 280.4 (57%) |
| 282.5 (22%) | 281.8 (60%) | 282.0 (53%) | 282.0 (36%) | 282.0 (36%) | |
| Mg 1s | 1304.9 | 1304.4 | 1304.2 | - | 1304.5 |
| Catalyst | GVL Yield,% |
|---|---|
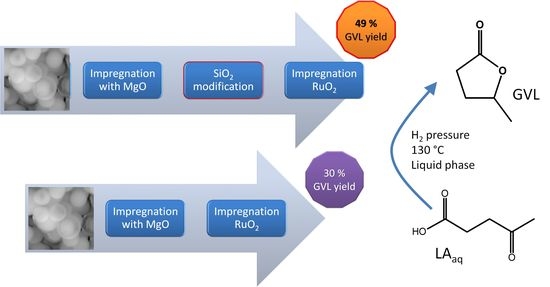
| Ru/NS/3Mg/NS | 49 ± 4.1 |
| Ru/NS/5Mg/NS | 41 ± 0.4 |
| Ru/NS/6Mg/NS | 24 ± 1.9 |
| Ru/NS | 35 ± 0.5 |
| Ru/4Mg/NS | 30 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzelak, K.; Trejda, M. Spherical Silica Modified with Magnesium and Ruthenium—Synthesis, Characterization and Catalytic Properties. Materials 2021, 14, 7378. https://doi.org/10.3390/ma14237378
Grzelak K, Trejda M. Spherical Silica Modified with Magnesium and Ruthenium—Synthesis, Characterization and Catalytic Properties. Materials. 2021; 14(23):7378. https://doi.org/10.3390/ma14237378
Chicago/Turabian StyleGrzelak, Kalina, and Maciej Trejda. 2021. "Spherical Silica Modified with Magnesium and Ruthenium—Synthesis, Characterization and Catalytic Properties" Materials 14, no. 23: 7378. https://doi.org/10.3390/ma14237378
APA StyleGrzelak, K., & Trejda, M. (2021). Spherical Silica Modified with Magnesium and Ruthenium—Synthesis, Characterization and Catalytic Properties. Materials, 14(23), 7378. https://doi.org/10.3390/ma14237378