Cotton Fabric Modified with a PAMAM Dendrimer with Encapsulated Copper Nanoparticles: Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
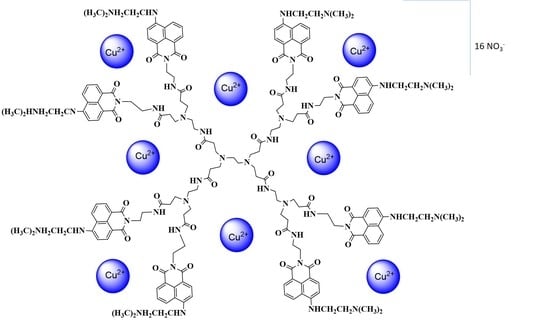
2.2. Synthesis of [Cu8(D)(NO3)16] Complex
2.3. Cotton Fabric Functionalization with Fluorescent PAMAM Dendrimer and Its Copper Complex
2.4. Colorimetric Analysis
2.5. Material Characterization
2.6. Antimicrobial Activity of Fabrics
3. Results
3.1. IR Characterization
3.2. Scanning Electron Microscope Analysis of Dendrimer D and [Cu8(D)(NO3)16]
3.3. EPR Characterization of the Dendrimer, Its Cu(II) Complex and Textile Samples Containing the Dendrimer, the Copper Complex, and the Dendrimer with Encapsulated Copper Nanoparticles
3.4. Scanning Electron Microscope Analysis of Cotton Fabric Treated with Dendrimer D and [Cu8(D)(NO3)10]
3.5. Comparative Colorimetric Study of the Cotton Fabric Dyed with Fluorescent Dendrimer, Its Copper Complex and after Impregnation with Aqueous Solution of the Reducing Agents (before and after Illumination with Visible Light)
3.6. Comparative Fluorescence Study of Cotton Fabric Dyed with Fluorescent Dendrimer, Its Copper Complex and after Its Impregnation with Aqueous Solution of Reducing Agents
3.7. Photo-Oxidation of Potassium Iodide
| Do + hν →1D | light excitation |
| 1D →3D | inter system crossing |
| 3D + 3O2 → Do + 1O2 | energy transfer |
| 1O2 + I− → IOO− → IOOH | |
| IOOH + I− → HOOI2− → I2 +HO2− → I3− + H2O2 + OH− | |
3.8. Antimicrobial Activity of Modified Cotton Fabrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Čongrádyová, A.; Jomová, K.; Kucková, L.; Kožíšek, J.; Monco, J.; Valko, M. Antimicrobial Activity of Copper (II) Complexes. J. Microbiol. Biotech. Food Sci. 2014, 3, 67. [Google Scholar]
- Ghosh, S.; Yadav, S.; Vasanthan, N.; Sekosan, G.A. A Study of Antimicrobial Property of Textile Fabric Treated with Modified Dendrimers. J. Appl. Polym. Sci. 2010, 115, 716. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- del Olmo, N.S.; Carloni, R.; Ortega, P.; García-Gallego, S.; de la Mata, F.J. Metallodendrimers as a promising tool in the biomedical field: An overview. Adv. Organomet. Chem. 2020, 74, 1–52. [Google Scholar]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Bio-Metallodendrimers—Emerging strategies in metal-based drug design. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, P.; Therrien, B.; Smith, G.S. Bio-Metallodendrimers–Emerging Strategies in Metal-Based Drug Design. Eur. J. Inorg. Chem. 2012, 17, 2853–2862. [Google Scholar]
- Scott, P. Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells; Simpson, D.H., Lo, K.K.-W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 205–243. [Google Scholar]
- Balogh, L.; Swanson, D.R.; Tomalia, D.A.; Hagnauer, G.L.; McManus, A.T. Dendrimer silver complexes and nanocomposites as antimicrobial agents. Nano Lett. 2001, 1, 18–21. [Google Scholar] [CrossRef]
- Suleman, N.; Kalhapure, R.S.; Mocktar, C.; Rambharose, S.; Singh, M.; Govender, T. Silver salts of carboxylic acid terminated generation 1 poly (propyl ether imine) (PETIM) dendron and dendrimers as antimicrobial agents against S. aureus and MRSA. RSC Adv. 2015, 5, 34967–34978. [Google Scholar] [CrossRef]
- Shaki, H.; Khosravi, A.; Gharanjig, K.; Mahboubi, A. Investigation of synthesis, characterization, photophysical and biological properties of novel antimicrobial fluorescent naphthalimide derivatives. Mater. Technol. 2016, 31, 322–331. [Google Scholar] [CrossRef]
- Mahltig, B.; Tatlises, B.; Fahmi, A.; Haase, H.J. Dendrimer stabilized silver particles for the antimicrobial finishing of textiles. J. Text. Inst. 2013, 104, 1042–1048. [Google Scholar] [CrossRef]
- Zhai, C.; Wei, C.; Xu, J.; Yang, P.; Du, Y. Synthesis and characterization of Au and Ag nanoparticles protected by PAMAM and its derivative. Colloid J. 2009, 71, 764–770. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Dzhardimalieva, G.I. Nanostructured Materials Preparation via Condensation Ways; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Barman, S.; Nain, A.; Jain, S.; Punjabi, N.; Mukherjib, S.; Satija, J. Dendrimer as a multifunctional capping agent for metal nanoparticles for use in bioimaging, drug delivery and sensor applications, J. Mater. Chem. B 2018, 6, 2368–2384. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Grabchev, I.; Vasileva-Tonkova, E.; Staneva, D.; Bosch, P.; Kukeva, R.; Stoyanova, R. Impact of Cu(II) and Zn(II) ions on the functional properties of new PAMAM metallodendrimers. New J. Chem. 2018, 42, 7853–7862. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Vasileva-Tonkova, E.; Alexandrova, R.; Cangiotti, M.; Fattori, A.; Ottaviani, M.F. Antimicrobial and anticancer activity of new poly(propyleneamine) metallodendrimers, J. Polym. Res. 2017, 24, 210. [Google Scholar] [CrossRef]
- Grabchev, I.; Yordanova, S.; Vasileva-Tonkova, E.; Bosch, P.; Stoyanov, S. Poly(propylenamine) dendrimers modified with 4-amino-1,8-naphthalimide: Synthesis, characterization and in vitro microbiological tests of their Cu(II) and Zn(II) complexes. Inorg. Chim. Acta 2015, 438, 179–188. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Bosch, P.; Vasileva-Tonkova, E.; Kukeva, R.; Stoyanova, R. Synthesis, characterisaion and antimicrobial activity of polypropylenamine metallodendrimers modified with 1,8-naphthalimides. J. Mol. Struct. 2018, 1164, 363–369. [Google Scholar] [CrossRef]
- Grabchev, I.; Staneva, D.; Vasileva-Tonkova, E.; Alexandrova, R. Antimicrobial and anticancer activity of fluorescent Zn(II) complexes of poly(propyleneamine) dendrimer modified with 1,8-naphthalimides. Chemosensors 2019, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Staneva, D.; Bosch, P.; Medel, S.; Grabchev, I. Evaluation of antimicrobial, biofilm inhibitory and cytotoxic activities of a new hiperbranched polymer modified with 1,8-naphthalimide units. Biointerface Res. Appl. Chem. 2018, 8, 3053–3059. [Google Scholar]
- Yordanova, S.; Grabchev, I.; Stoyanov, S.; Petkov, I. New detectors for metal cations and protons based on PAMAM dendrimers modified with 1,8-naphthalimide units. J. Photochem. Photobiol. A Chem. 2014, 283, 1–7. [Google Scholar] [CrossRef]
- Džimbeg-Malcic, V.; Barbaric-Mikocevic, Ž.; Itric, K. Kubelka-Munk theory in describing optical properties of paper. Tech. Gaz. 2011, 18, 117–124. [Google Scholar]
- Becerir, B. An Approach for Estimating the Relation between K/S Values and Dye Uptake. Colourage 2003, 50, 39–48. [Google Scholar]
- Makowski, T. Hydrophobization of cotton fabric with silanes with different substituents. Cellulose 2020, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. Int. J. Polym. Anal. Charact. 2017, 22, 104–111. [Google Scholar] [CrossRef]
- Mosinger, J.; Mosinger, B. Photodynamic sensitizers assay: Rapid and sensitive iodometric measurement. Experientia 1995, 51, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Yiming, X. Iodine-sensitized oxidation of ferrous ions under UV and visible light: The influencing factors and reaction mechanism. Photochem. Photobiol. Sci. 2013, 12, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Manov, H.; Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Nikolova, I.; Stoyanov, S.; Grabchev, I.J. Photosensitive dendrimers as a good alternative to antimicrobial photodynamic therapy of Gram-negative bacteria. Photochem. Photobiol. A Chem. 2021, 419, 113480. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Bosch, P.; Grozdanov, P.; Grabchev, I. Synthesis and Characterization of a New PAMAM Metallodendrimer for Antimicrobial Modification of Cotton Fabric. Macromol. Res. 2018, 26, 332–340. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Abdullah, H.; Kuo, D.-H. Photocatalytic antibacterial activity of copper-based nanoparticles under visible light illumination, J. Phys. Conf. Ser. 2018, 1007, 012062. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grozdanov, P.; Vilhelmova-Ilieva, N.; Nikolova, I.; Grabchev, I. Synthesis and photophysical characterisation of 3-bromo-4-dimethylamino-1,8-naphthalimides and their evaluation as agents for antibacterial photodynamic therapy. J. Photochem. Photobiol. A Chem. 2020, 401, 112730. [Google Scholar] [CrossRef]










| Sample Variant | Treatment | |||||
|---|---|---|---|---|---|---|
| Concentration of Dendrimer or Its Complex owf% | Concentration of Reductant owf% | Illumination with Visible Light | ||||
| No. | Name | 0.5 | 1.0 | Eosin Y | MDEA | 3 h |
| 1 | D1 | X | - | - | - | - |
| 2 | DR1I | X | - | 0.01 | 0.56 | X |
| 3 | DR1N | X | - | 0.01 | 0.56 | - |
| 4 | D2 | - | X | - | - | - |
| 5 | DR2I | - | X | 0.18 | 9.50 | X |
| 6 | DR2N | - | X | 0.18 | 9.50 | - |
| 7 | C1 | X | - | - | - | - |
| 8 | CR1I | X | - | 0.01 | 0.56 | - |
| 9 | CR1N | X | - | 0.01 | 0.56 | X |
| 10 | C2 | - | X | - | - | - |
| 11 | CR2I | - | X | 0.18 | 9.50 | X |
| 12 | CR2N | - | X | 0.18 | 9.50 | - |
| L* | a* | b* | |
|---|---|---|---|
| Cotton fabric | 93.77 | −0.26 | 3.75 |
| D1 | 83.04 | 2.65 | 52.61 |
| C1 | 81.11 | 3.68 | 47.76 |
| DR1I | 79.75 | 7.03 | 43.51 |
| CR1I | 76.79 | 8.36 | 43.19 |
| D2 | 79.00 | 5.89 | 53.97 |
| C2 | 76.68 | 6.79 | 52.91 |
| DR2I | 73.01 | 9.36 | 44.94 |
| CR2I | 72.82 | 11.04 | 47.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staneva, D.; Atanasova, D.; Nenova, A.; Vasileva-Tonkova, E.; Grabchev, I. Cotton Fabric Modified with a PAMAM Dendrimer with Encapsulated Copper Nanoparticles: Antimicrobial Activity. Materials 2021, 14, 7832. https://doi.org/10.3390/ma14247832
Staneva D, Atanasova D, Nenova A, Vasileva-Tonkova E, Grabchev I. Cotton Fabric Modified with a PAMAM Dendrimer with Encapsulated Copper Nanoparticles: Antimicrobial Activity. Materials. 2021; 14(24):7832. https://doi.org/10.3390/ma14247832
Chicago/Turabian StyleStaneva, Desislava, Daniela Atanasova, Ani Nenova, Evgenia Vasileva-Tonkova, and Ivo Grabchev. 2021. "Cotton Fabric Modified with a PAMAM Dendrimer with Encapsulated Copper Nanoparticles: Antimicrobial Activity" Materials 14, no. 24: 7832. https://doi.org/10.3390/ma14247832
APA StyleStaneva, D., Atanasova, D., Nenova, A., Vasileva-Tonkova, E., & Grabchev, I. (2021). Cotton Fabric Modified with a PAMAM Dendrimer with Encapsulated Copper Nanoparticles: Antimicrobial Activity. Materials, 14(24), 7832. https://doi.org/10.3390/ma14247832