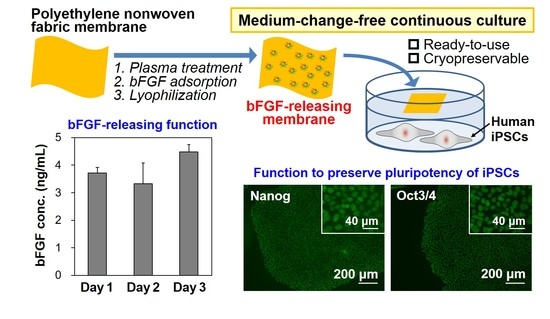
Storable bFGF-Releasing Membrane Allowing Continuous Human iPSC Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials for Preparation of bFGF-Releasing Membranes
2.2. Preparation of bFGF-Releasing Membranes
2.3. Lyophilization and Cryopreservation
2.4. Physicochemical Analysis
2.5. Preliminary bFGF-Release Test Using Acellular Medium
2.6. bFGF-Release Test Using iPSC-Containing Medium
2.7. Analysis of Cultured iPSCs
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis
3.2. Preliminary bFGF-Release Test to Examine the Effect of Lyophilization
3.3. Preliminary bFGF-Release Test to Examine the Effect of bFGF Adsorption Conditions
3.4. iPSC Culture Using the Lyophilized Membranes
3.5. Preliminary bFGF-Release Test to Examine the Effect of Cryopreservation
3.6. iPSC Culture Using Lyophilized and Cryopreserved Membranes
3.7. Mechanism and Function
3.8. Advantages of the Present bFGF-Releasing Membrane
3.9. Potential of the Present bFGF-Releasing Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, H.; Nagata, N.; Kurokawa, H.; Yamanaka, S. iPS cells: A game changer for future medicine. Embo J. 2014, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Engle, S.J.; Puppala, D. Integrating human pluripotent stem cells into drug development. Cell Stem Cell 2013, 12, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, F.M.; Driskell, R.R. The therapeutic potential of stem cells. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amit, M.; Carpenter, M.K.; Inokuma, M.S.; Chiu, C.-P.; Harris, C.P.; Waknitz, M.A.; Itskovitz-Eldor, J.; Thomson, J.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000, 227, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.-H.; Peck, R.M.; Li, D.S.; Feng, X.; Ludwig, T.; Thomson, J.A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods 2005, 2, 185–190. [Google Scholar] [CrossRef]
- Kinehara, M.; Kawamura, S.; Tateyama, D.; Suga, M.; Matsumura, H.; Mimura, S.; Hirayama, N.; Hirata, M.; Uchio-Yamada, K.; Kohara, A.; et al. Protein kinase C regulates human pluripotent stem cell self-renewal. PLoS ONE 2013, 8, e54122. [Google Scholar] [CrossRef] [Green Version]
- Furue, M.K.; Na, J.; Jackson, J.P.; Okamoto, T.; Jones, M.; Baker, D.; Hata, R.-I.; Moore, H.D.; Sato, J.D.; Andrews, P.W. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl. Acad. Sci. USA 2008, 105, 13409–13414. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Gulbranson, D.R.; Yu, P.; Hou, Z.; Thomson, J.A. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells 2012, 30, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Buchtova, M.; Chaloupkova, R.; Zakrzewska, M.; Vesela, I.; Cela, P.; Barathova, J.; Gudernova, I.; Zajickova, R.; Trantirek, L.; Martin, J.; et al. Instability restricts signaling of multiple fibroblast growth factors. Cell Mol. Life Sci. 2015, 72, 2445–2459. [Google Scholar] [CrossRef]
- Lotz, S.; Goderie, S.; Tokas, N.; Hirsch, S.E.; Ahmad, F.; Corneo, B.; Le, S.; Banerjee, A.; Kane, R.S.; Stern, J.H.; et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS ONE 2013, 8, e56289. [Google Scholar] [CrossRef] [Green Version]
- Han, U.; Park, H.H.; Kim, Y.J.; Park, T.H.; Park, J.H.; Hong, J. Efficient encapsulation and sustained release of basic fibroblast growth factor in nanofilm: Extension of the feeding cycle of human induced pluripotent stem cell culture. Acs Appl. Mater. Interf. 2017, 9, 25087–25097. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hong, J. Continuous release of bFGF from multilayer nanofilm to maintain undifferentiated human iPS cell cultures. Integr. Biol. 2014, 6, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2– A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zisch, A.H.; Lutolf, M.P.; Hubbell, J.A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 2003, 12, 295–310. [Google Scholar] [CrossRef]
- Davies, M.J.; Mitchell, C.A.; Maley, M.A.L.; Grounds, M.D.; Harvey, A.R.; Plant, G.W.; Wood, D.J.; Hong, Y.; Chirila, T.V. In vitro assessment of the biological activity of basic fibroblast growth factor released from various polymers and biomatrices. J. Biomater. Appl. 1997, 12, 31–56. [Google Scholar] [CrossRef]
- Gallagher, J.T.; Turnbull, J.E. Heparan sulphate in the binding and activation of basic fibroblast growth factor. Glycobiology 1992, 2, 523–528. [Google Scholar] [CrossRef]
- Oyane, A.; Araki, H.; Nakamura, M.; Aiki, Y.; Higuchi, K.; Pyatenko, A.; Adachi, M.; Ito, Y. Controlled release of basic fibroblast growth factor from a water-floatable polyethylene nonwoven fabric sheet for maintenance culture of iPSCs. Rsc Adv. 2020, 10, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Roy, I.; Gupta, M.N. Freeze-drying of proteins: Some emerging concerns. Biotechnol. Appl. Biochem. 2004, 39, 165–177. [Google Scholar] [CrossRef]
- Guruvenket, S.; Rao, G.M.; Komath, M.; Raichur, A.M. Plasma surface modification of polystyrene and polyethylene. Appl. Surf. Sci. 2004, 236, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Lehocký, M.; Drnovská, H.; Lapčíková, B.; Barros-Timmons, A.M.; Trindade, T.; Zembala, M.; Lapčík, L., Jr. Plasma surface modification of polyethylene. Colloids Surf. A Phys. Eng. Asp. 2003, 222, 125–131. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onuma, Y.; Higuchi, K.; Aiki, Y.; Shu, Y.; Asada, M.; Asashima, M.; Suzuki, M.; Imamura, T.; Ito, Y. A stable chimeric fibroblast growth factor (FGF) can successfully replace basic FGF in human pluripotent stem cell culture. PLoS ONE 2015, 10, e0118931. [Google Scholar] [CrossRef] [PubMed]
- Onuma, Y.; Tateno, H.; Hirabayashi, J.; Ito, Y.; Asashima, M. rBC2LCN, a new probe for live cell imaging of human pluripotent stem cells. Biochem. Biophys. Res. Commun. 2013, 431, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestrelski, S.J.; Tedeschi, N.; Arakawa, T.; Carpenter, J.F. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys. J. 1993, 65, 661–671. [Google Scholar] [CrossRef] [Green Version]
- D’Amore, P.A. Modes of FGF release in vivo and in vitro. Canc. Metastasis Rev. 1990, 9, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Masui, S.; Nakatake, Y.; Toyooka, Y.; Shimosato, D.; Yagi, R.; Takahashi, K.; Okochi, H.; Okuda, A.; Matoba, R.; Sharov, A.A.; et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007, 9, 625–635. [Google Scholar] [CrossRef]
- Chambers, I.; Silva, J.; Colby, D.; Nichols, J.; Nijmeijer, B.; Robertson, M.; Vrana, J.; Jones, K.; Grotewold, L.; Smith, A. Nanog safeguards pluripotency and mediates germline development. Nature 2007, 450, 1230–1235. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Li, J.; Li, D.; Xiao, C.; Xiao, H.; Yang, H.; Zhuang, X.; Chen, X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019, 90, 1–34. [Google Scholar] [CrossRef]
- Trevisol, T.C.; Langbehn, R.K.; Battiston, S.; Immich, A.P.S. Nonwoven membranes for tissue engineering: An overview of cartilage, epithelium, and bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1026–1049. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyane, A.; Araki, H.; Nakamura, M.; Aiki, Y.; Ito, Y. Storable bFGF-Releasing Membrane Allowing Continuous Human iPSC Culture. Materials 2021, 14, 651. https://doi.org/10.3390/ma14030651
Oyane A, Araki H, Nakamura M, Aiki Y, Ito Y. Storable bFGF-Releasing Membrane Allowing Continuous Human iPSC Culture. Materials. 2021; 14(3):651. https://doi.org/10.3390/ma14030651
Chicago/Turabian StyleOyane, Ayako, Hiroko Araki, Maki Nakamura, Yasuhiko Aiki, and Yuzuru Ito. 2021. "Storable bFGF-Releasing Membrane Allowing Continuous Human iPSC Culture" Materials 14, no. 3: 651. https://doi.org/10.3390/ma14030651
APA StyleOyane, A., Araki, H., Nakamura, M., Aiki, Y., & Ito, Y. (2021). Storable bFGF-Releasing Membrane Allowing Continuous Human iPSC Culture. Materials, 14(3), 651. https://doi.org/10.3390/ma14030651