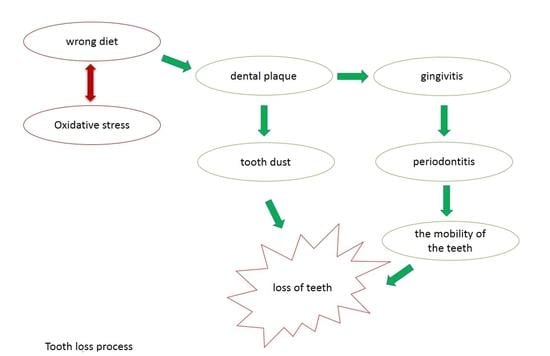
The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity
Abstract
:1. Introduction
1.1. Oral Cavity-Its Specificity in Relation to the Human Body
1.2. Aim of the Work
- (1)
- estimation of the influence of food on the induction of inflammation of the soft tissues in the oral cavity in the presence of residual biofilm
- (2)
- estimation of the influence of food on the inflammation of soft tissues in the oral cavity in the absence of residual biofilm
- (3)
- to estimate the effect of food on supra- and subgingival biofilm: is it the same or different?
- (4)
- to analyze the types of food in terms of the possibility of slowing down the development of inflammation in the oral cavity or rejecting the above possibility
- (5)
- to estimate which products cause the greatest oxidative stress in the oral cavity of humans.
- -
- periodontitis
- -
- necrotic periodontal diseases
- -
- periodontitis as a symptom of systemic diseases.
- -
- smoking
- -
- variety of diet
- -
- nutritional deficiencies, e.g., vitamin C deficiency
- -
- hormonal changes such as maturation
- -
- diabetes.
- -
- No changes around the implant,
- -
- Inflammation of the mucosa around the implant,
- -
- Peri-implantitis,
- -
- The loss of soft and hard tissues around the implant.
1.3. Oral Microflora Colonization
1.4. The Colonization of Microorganisms on the Tooth Surface
1.5. Bacterial Biofilm-Oral Health and Inflammation
1.6. Inflammation in the Oral Cavity
1.7. Oxidative Stress—A Stimulator of Inflammation in Oral Cavity
1.8. Oxidative Stress and Periodontitis
2. Diet—The Basis of Human Health and Inflammation
2.1. Diet
2.1.1. Collagen
2.1.2. Coenzyme Q10
2.1.3. Catechins
2.1.4. Vitamin C
2.1.5. β-Carotene
2.1.6. Omega-3
2.1.7. Fungotherapy Beneficial for Periodontium
2.2. Diet and Oral Health
- Diet F including meals, containing proteins, carbohydrates-sugars, fats, vegetables.
- Diet B. Mainly targeted at protein products. You can eat other foods as well, but end each meal with a sugar-free protein product such as kefir, yoghurt, cheese, etc.
- Diet W. Mainly oriented towards vegetables and other foods can also be eaten, but each meal should end with vegetables, such as radish, watercress, kale, broccoli, kohlrabi, etc.
- Diet T. Mainly targeted at foods containing Omega-3 fatty acids, can also eat other foods, but each meal should be finished with food containing Omega-3 fats, e.g., fish-especially salmon, herring, mackerel, sardines, seafood, sushi, rapeseed oil, linseed, soybean oil, soy products, nuts, almonds, pumpkin seeds.
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| A. actinomycetemcomitans | Aggregatibacter actinomycetemcomitans |
| P. gingivalis | Porphyromonas gingivalis |
| T. forsythia | Tannerella forsythia |
| T. denticola | Treponema denticola |
| F. nucleatum | Fusobacterium nucleatum |
| P. intermedia | Prevotella intermedia |
| L. salivarius | Ligilactobacillussalivarius |
| S. pyogenes | Streptococcus pyogenes |
| S. sanguinis | Streptococcus sanguinis |
| S. mutans | Streptococcus mutans |
| L. delbrueckii | Ligilactobacillusdelbrueckii |
| E. corrodens | Eikenella corrodens |
| C. concisus | Campylobacter concisus |
| V. parvula | Veillonella parvula |
| A. odontolyticus | Actinomyces odontolyticus |
| P. micros | Peptostreptococcus micros |
| E. nodatum | Eubacterium nodatum |
| S. constellatus | Streptococcus constellatus |
References
- Madani, M.; Berardi, T.; Stoopler, E.T. Anatomic and examination considerations of the oral cavity. Med. Clin. N. Am. 2014, 98, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, H. Biomolecules and biomarkers in oral cavity: Bioassays and immunopathology. J. Immunoass. Immunochem. 2019, 40, 52–69. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox Biology of Respiratory Viral Infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, F.A.F.; Forte, F.C.P.; Silva, P.G.B.; Lopes, C.B.; Montenegro, R.C.; Dos Santos, Â.K.C.R.; Mota, M.R.L.; Sousa, F.B.; Alves, A.P.N.N. Relationship of Streptococcus mutans with valvar cardiac tissue: A molecular and immunohistochemical study. J. Oral Pathol. Med. 2019, 48, 745–753. [Google Scholar] [CrossRef]
- Lamy, E.; Capela-Silva, F.; Tvarijonaviciute, A. Research on Saliva Secretion and Composition. Biomed. Res. Int. 2018, 2018, 7406312. [Google Scholar] [CrossRef] [Green Version]
- Pandit, P.; Cooper-White, J.; Punyadeera, C. High-yield RNA-extraction method for saliva. Clin Chem. 2013, 59, 1118–1122. [Google Scholar] [CrossRef] [Green Version]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and Flavor Perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879. [Google Scholar] [CrossRef]
- Lexner, M.O.; Blomqvist, S.; Dahlén, G.; Twetman, S. Microbiological profiles in saliva and supragingival plaque from caries-active adolescents before and after a short-term daily intake of milk supplemented with probiotic bacteria—A pilot study. Oral Health Prev. Dent. 2010, 8, 383–388. [Google Scholar]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontol 2000 2016, 70, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.J. Role of adhesion in microbial colonization of host tissues: A contribution of oral microbiology. J. Dent. Res. 1996, 75, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, S.K.; Sampathkumar, V.; Ramasubbu, N.; Paster, B.J.; Fine, D.H. Aggregatibacter actinomycetemcomitans colonization and persistence in a primate model. Proc. Natl. Acad. Sci. USA 2019, 116, 22307–22313. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.C.; Petrie, L.E.; Cox, G. Bacterial Anti-adhesives: Inhibition of Staphylococcus aureus Nasal Colonization. ACS Infect. Dis. 2019, 5, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Jeziorek, M.; Frej-Mądrzak, M.; Choroszy-Król, I. The influence of diet on gastrointestinal Candida spp. colonization and the susceptibility of Candida spp. to antifungal drugs. Rocz. Panstw. Zakl. Hig. 2019, 70, 195–200. [Google Scholar] [CrossRef]
- Almaguer-Flores, A.; Ximénez-Fyvie, L.A.; Rodil, S.E. Oral bacterial adhesion on amorphous carbon and titanium films: Effect of surface roughness and culture media. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 196–204. [Google Scholar] [CrossRef]
- Haukioja, A.; Yli-Knuuttila, H.; Loimaranta, V.; Kari, K.; Ouwehand, A.C.; Meurman, J.H.; Tenovuo, J. Oral adhesion and survival of probiotic and other lactobacilli and bifidobacteria in vitro. Oral Microbiol. Immunol. 2006, 21, 326–332. [Google Scholar] [CrossRef]
- Sheets, S.M.; Potempa, J.; Travis, J.; Casiano, C.A.; Fletcher, H.M. Gingipains from Porphyromonas gingivalis W83 induce cell adhesion molecule cleavage and apoptosis in endothelial cells. Infect. Immun. 2005, 73, 1543–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef]
- Kadowaki, T.; Baba, A.; Abe, N.; Takii, R.; Hashimoto, M.; Tsukuba, T.; Okazaki, S.; Suda, Y.; Asao, T.; Yamamoto, K. Suppression of pathogenicity of Porphyromonas gingivalis by newly developed gingipain inhibitors. Mol. Pharmacol. 2004, 66, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Cutler, C.W.; Kalmar, J.R.; Genco, C.A. Pathogenic strategies of the oral anaerobe Porhyromonas gingivalis. Trends Microbiol. 1995, 3, 45–51. [Google Scholar] [CrossRef]
- Perricone, C.; Colafrancesco, S.; Mazor, R.D.; Soriano, A.; Agmon-Levin, N.; Shoenfeld, Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: Unveiling the pathogenic, clinical and diagnostic aspects. J. Autoimmun. 2013, 47, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Chegini, N.; Shiverick, K.T.; Lamont, R.J. Localization of P. gingivalis in preterm delivery placenta. J. Dent. Res. 2009, 88, 575–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, S.; Senty, L.; Das, S.; Noe, J.C.; Munro, C.L.; Kitten, T. Identification of Virulence Determinants for Endocarditis in Streptococcus sanguinis by Signature-Tagged Mutagenesis. Infect. Immun. 2005, 73, 6064–6074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagiellonian Innovation Center. In Vitro Test Report: “Antibacterial Action of the Salistat Diet Supplement Containing Lactobacillus Salivarius SGL 03, Lactoferrin, GOS and Lemon and Rosemary Oils on Streptococcus Pyogenes, Streptococcus sanguinis and Streptococcus Mutans”. 2017. Available online: https://en.uj.edu.pl/research/innovation (accessed on 26 September 2020).
- Chaves, B.D.; Brashears, M.M.; Nightingale, K.K. Applications and safety considerations of Lactobacillus salivarius as a probiotic in animal and human health. J. Appl. Microbiol. 2017, 123, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teanpaisan, R.; Piwat, S.; Dahlèn, G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett. Appl. Microbiol. 2011, 53, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Mayanagi, G.; Kimura, M.; Nakaya, S.; Hirata, H.; Sakamoto, M.; Benno, Y.; Shimauchi, H. Probiotic effects of orally administered Lactobacillus salivarius WB21-containing tablets on periodontopathic bacteria: A double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Peridontol. 2009, 36, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Shimauchi, H.; Mayanagi, G.; Nakaya, S.; Minamibuchi, M.; Ito, Y.; Yamaki, K.; Hirata, H. Improvement of periodontal condition by probiotics with Lactobacillus salivarius WB21: A randomized, double-blind, placebo-controlled study. J. Clin Periodontol. 2008, 35, 897–905. [Google Scholar] [CrossRef]
- Neville, B.A.; O’Toole, P.W. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Future Microbiol. 2010, 5, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Pidutti, P.; Federici, F.; Brandi, J.; Manna, L.; Rizzi, E.; Marini, U.; Cecconi, D. Purification and characterization of ribosomal proteins L27 and L30 having antimicrobial activity produced by the Lactobacillus salivarius SGL 03. J. Appl. Microbiol. 2018, 124, 398–407. [Google Scholar] [CrossRef]
- Kucia, M.; Wietrak, E.; Szymczak, M.; Kowalczyk, P. Effect of LigiLactobacillus salivarius and Other Natural Components against Anaerobic Periodontal Bacteria. Molecules 2020, 25, 4519. [Google Scholar] [CrossRef]
- Testa, M.M.; de Valladares, R.; de Cardenas, I.L. Antagonistic interactions among Fusobacterium nucleatum and Prevotella intermedia with oral lactobacilli. Res. Microbiol. 2003, 154, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Madej, A.; Paprocki, D.; Szymczak, M.; Ostaszewski, R. Coumarin Derivatives as New Toxic Compounds to Selected K12, R1–R4 E. coli Strains. Materials 2020, 13, 2499. [Google Scholar] [CrossRef] [PubMed]
- Bunetel, L.; Tamanai-Shacoori, Z.; Martin, B.; Autier, B.; Guiller, A.; Bonnaure-Mallet, M. Interactions between oral commensal Candida and oral bacterial communities in immunocompromised and healthy children. J. Mycol Med. 2019, 29, 223–232. [Google Scholar] [CrossRef]
- Darveau, R.P.; Hajishengallis, G.; Curtis, M.A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.C.; Ko, Y.; Hong, S.D.; Kim, K.Y.; Lee, Y.H.; Chae, C.; Choi, Y. Presence of Porphyromonas gingivalis and plasma cell dominance in gingival tissues with periodontitis. Oral Dis. 2010, 16, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Darveau, R.P. Contribution of Porphyromonas gingivalis lipopolysacharide to periodontitis. Periodontology 2010, 54, 53–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Shibutani Windsor, L.J. Effects of Porphyromonas gingivalis on human gingival fibroblasts from healthy and inflamed tissues. J. Peridont. Res. 2008, 43, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Dół formularzaLee, E.S.; de Josselin de Jong, E.; Kim, B.I. Detection of dental plaque and its potential pathogenicity using quantitative light-induced fluorescence. J. Biophotonics 2019, 12, e201800414. [Google Scholar] [CrossRef]
- Akifusa, S.; Isobe, A.; Kibata, K.; Oyama, A.; Oyama, H.; Ariyoshi, W.; Nishihara, T. Comparison of dental plaque reduction after use of electric toothbrushes with and without QLF-D-applied plaque visualization: A 1-week randomized controlled trial. BMC Oral Health 2020, 20, 4. [Google Scholar] [CrossRef] [Green Version]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 7–15. [Google Scholar] [CrossRef] [PubMed]
- Velsko, I.M.; Fellows Yates, J.A.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Martinez, J.B.R.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial differences between dental plaque and historic dental calculus are related to oral biofilm maturation stage. Microbiome 2019, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Huang, X.; Zhou, X.; Li, M.; Ren, B.; Peng, X.; Cheng, L. Influence of Dental Prosthesis and Restorative Materials Interface on Oral Biofilms. Int. J. Mol. Sci. 2018, 19, 3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, D.J. Dental calculus: Recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits. Eur. J. Oral Sci. 1997, 105, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Hazen, S.P. Supragingival dental calculus. Periodontol 2000 1995, 8, 125–136. [Google Scholar] [CrossRef] [PubMed]
- De Geest, S.; Laleman, I.; Teughels, W.; Dekeyser, C.; Quirynen, M. Periodontal diseases as a source of halitosis: A review of the evidence and treatment approaches for dentists and dental hygienists. Periodontol 2000 2016, 71, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Lingström, P. Nutrition, dental caries and periodontal disease: A narrative review. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S79–S84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinanoff, N.; Palmer, C.A. Dietary determinants of dental caries and dietary recommendations for preschool children. J. Public Health Dent. 2000, 60, 197–206; discussion 207–209. [Google Scholar] [CrossRef] [PubMed]
- Almusawi, M.A.; Gosadi, I.; Abidia, R.; Almasawi, M.; Khan, H.A. Potential risk factors for dental caries in Type 2 diabetic patients. Int. J. Dent. Hyg. 2018, 16, 467–475. [Google Scholar] [CrossRef]
- Slavkin, H.C. Biofilms: Microbial ecology and Antoni van leeuwenhoek. J. Am. Dent. Assoc. 1997, 128, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Ammons, M.C.; Copié, V. Lactoferrin: A bioinspired, anti-biofilm therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Rudolf, J.L.; Moser, C.; Sculean, A.; Eick, S. In-vitro antibiofilm activity of chlorhexidine digluconate on polylactide-based and collagen-based membranes. BMC Oral Health 2019, 19, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Avila, E.D.; Lima, B.P.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zijnge, V.; van Leeuwen, M.B.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J. Oral biofilm archit on natural teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, K.; Maruyama, M.; Uchiyama, A.; Shibasaki, K. Characterisation of a sucrose-independent in vitro biofilm model of supragingival plaque. Oral Dis. 2018, 24, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Signori, C.; Hartwig, A.D.; Silva-Júnior, I.F.D.; Correa, M.B.; Azevedo, M.S.; Cenci, M.S. The role of human milk and sucrose on cariogenicity of microcosm biofilms. Braz. Oral Res. 2018, 32, e109. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J. Cytokine regulation of immune responses to Porphyromonas gingivalis. Periodontology 2010, 54, 160–194. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.C.; Kesavalu, L.; Walker, S.; Genco, C.A. Virulence factors of Porphyromonas gingivalis. Periodontology 1999, 20, 168–238. [Google Scholar] [CrossRef] [PubMed]
- Scheres, N.; Crielaard, W. Gingival fibroblast responsiveness is differentially af-fected by Porphyromonas gingivalis: Implications for the pathogenesis of periodontitis. Mol. Oral Microbiol. 2012, 12, 1–15. [Google Scholar]
- Baris, O.; Demir, T.; Gulluce, M.; Niger, J. Investigation of In vitro Mineral forming bacterial isolates from supragingival calculus. Clin. Pr. 2017, 20, 1571–1575. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yip, H.K. Supragingival calculus: Formation and control. Crit. Rev. Oral Biol. Med. 2002, 13, 426–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haffajee, A.D.; Teles, R.P.; Socransky, S.S. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontology 2000, 42, 219–258. [Google Scholar] [CrossRef]
- Arredondo, A.; Blanc, V.; Mor, C.; Nart, J.; León, R. Resistance to beta-lactams and distribution of beta-lactam resistance genes in subgingival microbiota from Spanish patients with periodontitis. Clin. Oral Investig. 2020, 24, 4639–4648. [Google Scholar] [CrossRef]
- Ranganathan, A.T.; Sarathy, S.; Chandran, C.R.; Iyan, K. Subgingival prevalence rate of enteric rods in subjects with periodontal health and disease. J. Indian Soc. Periodontol. 2017, 21, 224–228. [Google Scholar] [CrossRef]
- Roberts-Harry, E.A.; Clerehugh, V. Subgingival calculus: Where are we now? A comparative review. J. Dent. 2000, 28, 93–102. [Google Scholar] [CrossRef]
- Shakibaie, F.; Law, K.; Walsh, L.J. Improved detection of subgingival calculus by laser fluorescence over differential reflectometry. Lasers Med. Sci. 2019, 34, 1807–1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corraini, P.; López, R. Reliability of recordings of subgingival calculus detected using an ultrasonic device. Clin. Oral Investig. 2015, 19, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Badran, Z.; Demoersman, J.; Struillou, X.; Boutigny, H.; Weiss, P.; Soueidan, A. Laser-induced fluorescence for subgingival calculus detection: Scientific rational and clinical application in periodontology. Photomed. Laser. Surg. 2011, 29, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa, M.; Aoki, A.; Kakizaki, S.; Taniguchi, Y.; Ejiri, K.; Mizutani, K.; Koshy, G.; Akizuki, T.; Oda, S.; Sumi, Y.; et al. In vitro and clinical evaluation of optical coherence tomography for the detection of subgingival calculus and root cementum. J. Oral Sci. 2018, 60, 418–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laleman, I.; Cortellini, S.; De Winter, S.; Rodriguez, H.E.; Dekeyser, C.; Quirynen, M.; Teughels, W. Subgingival debridement: End point, methods and how often? Periodontol 2000 2017, 75, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Beikler, T. Halitosis: The multidisciplinary approach. Int. J. Oral Sci. 2012, 4, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzoman, H. The association between periodontal diseases and halitosis among Saudi patients. Saudi Dent. J. 2021, 33, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Farag, N.S.; Breitinger, U.; Breitinger, H.G.; El Azizi, M.A. Viroporins and inflammasomes: A key to understand virus-induced inflammation. Int. J. Biochem. Cell Biol. 2020, 122, 105738. [Google Scholar] [CrossRef]
- Lewis, J.D.; Chen, E.Z.; Baldassano, R.N.; Otley, A.R.; Griffiths, A.M.; Lee, D.; Bittinger, K.; Bailey, A.; Friedman, E.S.; Hoffmann, C.; et al. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host Microbe 2015, 18, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; Von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoni, R.; Fontana, E.; Guglielmetti, S.; Fosso, B.; D’Erchia, A.M.; Maina, V.; Taverniti, V.; Castiello, M.C.; Mantero, S.; Pacchiana, G.; et al. Intestinal microbiota sustains inflammation and autoimmunity induced by hypomorphic RAG defects. J. Exp. Med. 2016, 213, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Picchianti-Diamanti, A.; Rosado, M.M.; D’Amelio, R. Infectious Agents and Inflammation: The Role of Microbiota in Autoimmune Arthritis. Front. Microbiol. 2018, 8, 2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.W.; Wang, X. Mobile microbiome: Oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013, 92, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yan, A.; Liu, X.; Ma, Y.; Zhao, F.; Wang, M.; Loor, J.J.; Wang, H. Melatonin ameliorates ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J. Hazard. Mater. 2021, 407, 124489. [Google Scholar] [CrossRef] [PubMed]
- Miggiano, G.A.; Gagliardi, L. Diet, nutrition and rheumatoid arthritis. Clin. Ter. 2005, 156, 115–123. [Google Scholar] [PubMed]
- Watad, A.; Quaresma, M.; Bragazzi, N.L.; Cervera, R.; Tervaert, J.W.C.; Amital, H.; Shoenfeld, Y. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)/Shoenfeld’s syndrome: Descriptive analysis of 300 patients from the international ASIA syndrome registry. Clin. Rheumatol. 2018, 37, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Dzidic, M.; Collado, M.C.; Abrahamsson, T.; Artacho, A.; Stensson, M.; Jenmalm, M.C.; Mira, A. Oral microbiome development during childhood: An ecological succession influenced by postnatal factors and associated with tooth decay. ISME J. 2018, 12, 2292–2306. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, S.; Mullan, F. The role of the diet in tooth wear. Br. Dent. J. 2018, 224, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, M.; Giovannucci, E.; Krall Kaye, E.; Joshipura, K.J.; Dietrich, T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014, 17, 844–852. [Google Scholar] [CrossRef] [Green Version]
- Simpson, T.C.; Weldon, J.C.; Worthington, H.V.; Needleman, I.; Wild, S.H.; Moles, D.R.; Stevenson, B.; Furness, S.; Iheozor-Ejiofor, Z. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015, 11, CD004714. [Google Scholar] [CrossRef]
- Winning, L.; Linden, G.J. Periodontitis and systemic disease. BDJ Team 2015, 2. [Google Scholar] [CrossRef]
- Kim, J.; Amar, S. Odontology. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology 2006, 94, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arigbede, A.O.; Babatope, B.O.; Bamidele, M.K. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012, 16, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Nędzi-Góra, M.; Kowalski, J.; Krajewski, J.; Górska, R. Microbiological analysis of deep periodontal pockets in people with chronic periodontitis by PCR. Stomatol. J. 2007, 11, 717–725. [Google Scholar]
- Sbordone, L.; DiGenio, M.; Bortolaia, C. Bacterial virulence in the etiology of periodontal diseases. Minerva Stomatol. 2000, 49, 485–500. [Google Scholar]
- Sela, M.N. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. Med. 2001, 12, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M. Aggressive and acute periodontal diseases. Periodontology 2000 2014, 65, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Colombo, A.V.; Silva, C.M.; Haffajee, A.; Colombo, A.P.V. Identification of oral bacteria associated with crevicular epithelial cells from chronic periodontitis lesions. J. Med. Microbiol. 2006, 55, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A.; Dietrich, T.; Garcia, R.I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc. 2010, 58, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M.; et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech. Dis. 2017, 6, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, S.-H.; Kang, S.-H.; Yoon, C.-H.; Lee, H.-J.; Yun, P.-Y.; Youn, T.-J.; Chae, I.-H. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: A population-based study from Korea. Eur. Heart J. 2019, 40, 1138–1145. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. The Bidirectional Interactions between Resveratrol and Gut Microbiota: An Insight into Oxidative Stress and Inflammatory Bowel Disease Therapy. Biomed. Res. Int. 2019, 2019, 5403761. [Google Scholar] [CrossRef] [PubMed]
- Dursun, E.; Akalin, F.A.; Genc, T.; Cinar, N.; Erel, O.; Yildiz, B.O. Oxidative Stress and Periodontal Disease in Obesity. Medicine 2016, 95, e3136. [Google Scholar] [CrossRef] [PubMed]
- Buranasin, P.; Mizutani, K.; Iwasaki, K.; Pawaputanon, N.; Mahasarakham, C.; Kido, D.; Takeda, K.; Izumi, Y. High glucose-induced oxidative stress impairs proliferation and migration of human gingival fibroblasts. PLoS ONE 2018, 13, e0201855. [Google Scholar] [CrossRef] [PubMed]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Comparative In Vitro Resistance of Human Periodontal Bacterial Pathogens to Tinidazole and Four Other Antibiotics. Antibiotics 2020, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Fan, C.; Guo, L.; Gu, H.; Huo, Y.; Lin, H. Alterations in Oral-Nasal-Pharyngeal Microbiota and Salivary Proteins in Mouth-Breathing Children. Front. Microbiol. 2020, 11, 575550. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zheng, W.; Cai, Q.Y.; Shrubsole, M.J.; Pei, Z.; Brucker, R.; Steinwandel, M.D.; Bordenstein, S.R.; Li, Z.; Blot, W.J.; et al. Cigarette smoking and oral microbiota in low-income and African-American populations. J. Epidemiol. Community Health 2019, 73, 1108–1115. [Google Scholar] [CrossRef]
- Yu, G.; Phillips, S.; Gail, M.H.; Goedert, J.J.; Humphrys, M.S.; Ravel, J.; Ren, Y.; Caporaso, N.E. The effect of cigarette smoking on the oral and nasal microbiota. Microbiome 2017, 5, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grine, G.; Terrer, E.; Boualam, M.A.; Aboudharam, G.; Chaudet, H.; Ruimy, R.; Drancourt, M. Tobacco-smoking-related prevalence of methanogens in the oral fluid microbiota. Sci. Rep. 2018, 8, 9197. [Google Scholar] [CrossRef] [PubMed]
- Boqué, N.; Campión, J.; Milagro, F.I.; Moreno-Aliaga, M.J.; Martinez, J.A. Some cyclin-dependent kinase inhibitors-related genes are regulated by vitamin C in a model of diet-induced obesity. Biol. Pharm. Bull. 2009, 32, 1462–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacaman, R.A. Sugars and beyond. The role of sugars and the other nutrients and their potential impact on caries. Oral Dis. 2018, 24, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; Pompili, M.; Di Stasio, E.; Zocco, M.A.; Gasbarrini, A.; Flore, R. Subclinical atherosclerosis is linked to small intestinal bacterial overgrowth via vitamin K2-dependent mechanisms. World J. Gastroenterol. 2017, 23, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D(3) Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.N.; Gaby, S.K. Premalignant lesions: Role of antioxidant vitamins and beta-carotene in risk reduction and prevention of malignant transformation. Am. J. Clin. Nutr. 1991, 53, 386S–390S. [Google Scholar] [CrossRef]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing Skin Health: By Oral Administration of Natural Compounds and Minerals with Implications to the Dermal Microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef] [Green Version]
- Magán-Fernández, A.; Papay-Ramírez, L.; Tomás, J.; Marfil-Álvarez, R.; Rizzo, M.; Bravo, M.; Mesa, F. Association of simvastatin and hyperlipidemia with periodontal status and bone metabolism markers. J. Periodontol. 2014, 85, 1408–1415. [Google Scholar] [CrossRef]
- Bartold, P.M. Turnover in periodontal connective tissues: Dynamic homeostasis of cells, collagen and ground substances. Oral Dis. 1995, 1, 238–253. [Google Scholar] [CrossRef]
- Manthena, S.; Rao, M.V.; Penubolu, L.P.; Putcha, M.; Harsha, A.V. Effectiveness of CoQ10 Oral Supplements as an Adjunct to Scaling and Root Planing in Improving Periodontal Health. J. Clin. Diagn. Res. 2015, 9, ZC26–ZC28. [Google Scholar] [CrossRef]
- Yelins’ka, A.M.; Liashenko, L.I.; Kostenko, V.O. Quercetin potentiates antiradical properties of epigallocatechin-3-gallate in periodontium of rats under systemic and local administration of lipopolisaccharide of salmonella typhi. Wiad. Lek. 2019, 72, 1499–1503. [Google Scholar] [CrossRef]
- Shanmugam, T.; Selvaraj, M.; Poomalai, S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: An in-vivo and in-silico study. Int. Immunopharmacol. 2016, 39, 128–139. [Google Scholar] [CrossRef]
- Sriram, N.; Kalayarasan, S.; Sudhandiran, G. Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling. Pulm. Pharmacol. Ther. 2009, 22, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapple, I.L.C.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44, S39–S51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher Intakes of Fruits and Vegetables, beta-Carotene, Vitamin C, alpha-Tocopherol, EPA, and DHA Are Positively Associated with Periodontal Healing after Nonsurgical Periodontal Therapy in Nonsmokers but Not in Smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 17, 28. [Google Scholar] [CrossRef] [Green Version]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The influence of an anti-inflammatory diet on gingivitis. A randomized controlled trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef]
- Teshome, A.; Yitayeh, A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral Health 2016, 17, 31. [Google Scholar] [CrossRef] [Green Version]
- Belstrøm, D. The salivary microbiota in health and disease. J. Oral Microbiol. 2020, 12, 1723975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, H.D.; Park, S.; Oh, K.; Kim, H.J.; Shin, H.R.; Moon, H.K.; Kim, J. Diet and cancer risk in the Korean population: A meta- analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8509–8519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary mushroom intake may reduce the risk of breast cancer: Evidence from a meta-analysis of observational studies. PLoS ONE 2014, 9, e93437. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P.; Stephen, A.S. Use of Probiotics and Oral Health. Curr. Oral Health Rep. 2017, 4, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18. [Google Scholar]
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Microbial complexes in supragingival plaque. Oral Microbiol. Immunol. 2008, 23, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, P.; Chatterjee, A.; Raghunathan, V. Probiotics in the treatment of periodontal disease: A systematic review. J. Indian Soc. Periodontol. 2016, 20, 488–495. [Google Scholar] [CrossRef]
- Food and Agriculture Organization, World Health Organization. Guidelines for the Evaluation of Probiotics in Food—Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization: Rome, Italy; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Stamatova, I.; Meurman, J.H. Probiotics and periodontal disease. Periodontology 2009, 51, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.; Lee, S.; Park, H.; Shin, H.; Holzapfel, W.; Huh, C.S. Development of putative probiotics as feed additives: Validation in a porcine-specific gas-trointestinal tract model. Appl. Microbiol. Biotechnol. 2016, 100, 10043–10054. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; La Vecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef]
- Garavello, W.; Lucenteforte, E.; Bosetti, C.; La Vecchia, C. The role of foods and nutrients on oral and pharyngeal cancer risk. Minerva Stomatol. 2009, 58, 25–34. [Google Scholar] [PubMed]
- Moynihan, P.J. The role of diet and nutrition in the etiology and prevention of oral diseases. Bull World Health Organ. 2005, 83, 694–699. [Google Scholar] [PubMed]
- Lavin, J.H.; French, S.J.; Ruxton, C.H.; Read, N.W. An investigation of the role of oro-sensory stimulation in sugar satiety? Int. J. Obes. Relat. Metab. Disord. 2002, 26, 384–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesalski, H.K. (Ed.) Pocket Atlas of Nutrition, 1st ed.; Grimm Medical Books; Georg Thieme Verlag: Stuttgart, Germany, 2004; ISBN-13: 978-3131354815, ISBN-10: 9783131354815. [Google Scholar]
- Konopka, T.; Dembowska, E.; Pietruska, M.; Dymalski, P.; Górska, R. Periodontal status and selected parameters of oral condition of Poles aged 65 to 74 years. Przegl. Epidemiol. 2015, 69, 537–542. [Google Scholar]
- Lee, W.H.; Chen, H.M.; Yang, S.F.; Liang, C.; Peng, C.Y.; Lin, F.M.; Tsai, L.L.; Wu, B.C.; Hsin, C.H.; Chuang, C.Y.; et al. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Angelis, M.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef] [PubMed]
- Rachid, R.; Chatila, T.A. The role of the gut microbiota in food allergy. Curr. Opin. Pediatr. 2016, 28, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The oral microbiota—A mechanistic role for systemic diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhatalo, A.; Blackwell, J.R.; L’Heureux, J.E.; Williams, D.W.; Smith, A.; van der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Molfino, A.; Testorio, M.; Perrotta, A.M.; Currado, A.; Pintus, G.; Pietrucci, D.; Unida, V.; La Rocca, D.; Biocca, S.; et al. Effect of Low-Protein Diet and Inulin on Microbiota and Clinical Parameters in Patients with Chronic Kidney Disease. Nutrients 2019, 11, 3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esberg, A.; Haworth, S.; Hasslöf, P.; Lif Holgerson, P.; Johansson, I. Oral Microbiota Profile Associates with Sugar Intake and Taste Preference Genes. Nutrients 2020, 12, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, I.; Vasquez, A.; Moyerbrailean, G.; Land, S.; Djuric, Z.; Sun, J.; Lin, H.-L.; Ram, J.L. Nutritional Correlates of Human Oral Microbiome. J. Am. Coll. Nutr. 2017, 36, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Chen, Z.; Han, S.; Zhou, D.; Zhou, S.; Jia, G. Effects of oral exposure to titanium dioxide nanoparticles on gut microbiota and gut-associated metabolism in vivo. Nanoscale 2019, 11, 22398–22412. [Google Scholar] [CrossRef]
- Marquis, R.E. Applied and ecological aspects of oxidative-stress damage to bacterial spores and to oral microbes. Sci. Prog. 2004, 87 Pt 3, 153–177. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. J. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postepy Hig. Med. Dosw. 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, F.; Mazur, M.; Ndokaj, A.; Corridore, D.; La Torre, G.; Polimeni, A.; Ottolenghi, L. Periodontitis and the microbiome: A systematic review and meta-analysis. Minerva Stomatol. 2018, 67, 250–258. [Google Scholar] [CrossRef]
- Kumar, V.; Farell, G.; Yu, S.; Harrington, S.; Fitzpatrick, L.; Rzewuska, E.; Miller, V.M.; Lieske, J.C. Cell biology of pathologic renal calcification: Contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J. Investig. Med. 2006, 54, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the oral microbiome in personalized dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Paju, S.; Pietiäinen, M.; Liljestrand, J.M.; Lahdentausta, L.; Salminen, A.; Kopra, E.; Mäntylä, P.; Buhlin, K.; Hörkkö, S.; Sinisalo, J.; et al. Carotid artery calcification in panoramic radiographs associates with oral infections and mortality. Int. Endod. J. 2021, 54, 15–25. [Google Scholar] [CrossRef]
- Krause, F.; Schmalz, G.; Park, K.J.; Schmidt, J.; Ziebolz, D.; Schneider, H.; Haak, R. Evaluation of calculus imaging on root surfaces by spectral-domain optical coherence tomography. Photodiagnosis Photodyn. 2019, 25, 275–279. [Google Scholar] [CrossRef]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.B.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2017, 67, 1454–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.; Pérez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontology 2000 2005, 38, 135–187. [Google Scholar] [CrossRef] [PubMed]
- WHO Report Guideline: Sugars Intake for Adults and Children. 4 March 2015. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 14 March 2021).
- Tennert, C.; Reinmuth, A.C.; Bremer, K.; Al-Ahmad, A.; Karygianni, L.; Hellwig, E.; Vach, K.; Ratka-Krüger, P.; Wittmer, A.; Woelber, J.P. An oral health optimized diet reduces the load of potential cariogenic and periodontal bacterial species in the supragingival oral plaque: A randomized controlled pilot study. Microbiologyopen 2020, 9, e1056. [Google Scholar] [CrossRef]
- Sales-Peres, S.H.C.; de Azevedo-Silva, L.J.; Bonato, R.C.S.; Sales-Peres, M.C.; Pinto, A.C.S.; Santiago Junior, J.F. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: Meta-analysis of the epidemiological evidence. Meta-Anal. Obes. Res. Clin. Pract. 2020, 14, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Wang, H.L.; Romanos, G.E. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, I.S.; Cruz, S.S.D.; Trindade, S.C.; Passos-Soares, J.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis. 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, X.; Zou, X.; Zhang, Y.; Wang, J.; Wang, Y. Relationship between periodontal disease and lung cancer: A systematic review and meta-analysis. J. Periodontal Res. 2020, 55, 581–593. [Google Scholar] [CrossRef]
- Zeng, X.T.; Xia, L.Y.; Zhang, Y.G.; Li, S.; Leng, W.D.; Kwong, J.S. Periodontal Disease and Incident Lung Cancer Risk: A Meta-Analysis of Cohort Studies. J. Periodontol. 2016, 87, 1158–1164. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Jepsen, S.; Needleman, I.; Roldán, S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 136–159. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S.; Gunsolley, J.C. Systemic anti-infective periodontal therapy. A systematic review. Ann. Periodontol. 2003, 8, 115–181. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Alonso, B.; León, R.; Roldán, S.; Sanz, M. Antimicrobial therapy in periodontitis: The use of systemic antimicrobials against the subgingival biofilm. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 45–66. [Google Scholar] [CrossRef] [PubMed]
- Slot, D.E.; Dörfer, C.E.; Van der Weijden, G.A. The efficacy of interdental brushes on plaque and parameters of periodontal inflammation: A systematic review. Int. J. Dent. Hyg. 2008, 6, 253–264. [Google Scholar] [CrossRef]
- da Silva, F.R.P.; Pessoa, L.D.S.; Vasconcelos, A.C.C.G.; de Aquino Lima, W.; Alves, E.H.P.; Vasconcelos, D.F.P. Polymorphisms in interleukins 17A and 17F genes and periodontitis: Results from a meta-analysis. Mol. Biol. Rep. 2017, 44, 443–453. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Singh, P.; Ajmera, D.H.; Song, J.; Ji, P. Association of Common Variants in MMPs with Periodontitis Risk. Dis. Mark. 2016, 2016, 1545974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, F.R.P.; Vasconcelos, A.C.C.G.; de Carvalho França, L.F.; Di Lenardo, D.; Nascimento, H.M.S.; Vasconcelos, D.F.P. Association between the rs1143634 polymorphism in interleukin-1B and chronic periodontitis: Results from a meta-analysis composed by 54 case/control studies. Gene 2018, 668, 97–106. [Google Scholar] [CrossRef]
- Karimbux, N.Y.; Saraiya, V.M.; Elangovan, S.; Allareddy, V.; Kinnunen, T.; Kornman, K.S.; Duff, G.W. Interleukin-1 gene polymorphisms and chronic periodontitis in adult whites: A systematic review and meta-analysis. J. Periodontol. 2012, 83, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Claffey, N. European Workshop in Periodontology group C. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 210–213. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pihlstrom, B.l.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Gendron, R.; Grenier, D.; Maheu-Robert, L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000, 2, 897–906. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef] [Green Version]
- Gemmell, E.; Marshall, R.I.; Seymour, G.J. Cytokines and prostaglandins in immune homeostasis and tissue destruction in periodontal disease. Periodontology 2000 1997, 14, 112–143. [Google Scholar] [CrossRef]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival Crevicular as a Source of Biomarkers for Periodontitis. Periodontol 2000 2016, 70, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Classifying periodontal diseases—A long-standing dilemma. Periodontology 2000 2002, 30, 9–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, G.C. Analysis of gingival crevice fluid and risk of progression of periodontitis. Periodontology 2000 2004, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yamaguchi, Y.; Yoshitake, K.; Saeki, Y. Effects of TNFα and prostaglandin E2 on the expression of MMPs in human periodontal ligament fibroblasts. J. Periodontal. Res. 2002, 37, 167–176. [Google Scholar] [CrossRef]
- Bascones-Martinez, A.; Munoz-Corcuera, M.; Noronha, S.; Mota, P.; Bascones-Ilundain, C.; Campo-Trapero, J. Host defence mechanisms against bacterial aggression in periodontal disease: Basic mechanisms. Med. Oral Patol. Oral Cir. Bucal. 2009, 14, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Cai, W.; Zhao, S.; Shi, L.; Chen, Y.; Li, X.; Sun, X.; Mao, Y.; He, B.; Hou, Y.; et al. Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Abduljabbar, T.; Abu Hassan, M.I.; Javed, F.; Vohra, F. Cytokine Profile in Chronic Periodontitis Patients with and without Obesity: A Systematic Review and Meta-Analysis. Dis. Mark. 2016, 2016, 4801418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, A.F.; Angst, P.D.; Arce, R.M.; Gomes, S.C.; Oppermann, R.V.; Susin, C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: A meta-analysis. J. Clin. Periodontol. 2016, 43, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, Z.; Zhu, X.; Li, W.; Chen, J. Treating periodontitis—A systematic review and meta-analysis comparing ultrasonic and manual subgingival scaling at different probing pocket depths. BMC Oral Health 2020, 20, 176. [Google Scholar] [CrossRef]
- Akram, Z.; Rahim, Z.H.; Taiyeb-Ali, T.B.; Shahdan, M.S.; Baharuddin, N.A.; Vaithilingam, R.D.; Safii, S.H. Resistin as potential biomarker for chronic periodontitis: A systematic review and meta-analysis. Arch. Oral Biol. 2017, 73, 311–320. [Google Scholar] [CrossRef]
- Finoti, L.S.; Nepomuceno, R.; Pigossi, S.C.; Corbi, S.C.; Secolin, R.; Scarel-Caminaga, R.M. Association between interleukin-8 levels and chronic periodontal disease: A PRISMA-compliant systematic review and meta-analysis. Medicine 2017, 96, e6932. [Google Scholar] [CrossRef]
- Cox, S.W.; Eley, B.M.; Kiili, M.; Sikainen, A.; Tervahartiala, T.; Sorsa, T. Collagen degradation by interleukin-1β-stimulated gingival fibroblasts is accompanied by release and activation of multiple matrix metalloproteinases and cysteine proteinases. Oral Dis. 2006, 12, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gu, B.; Zhao, L.; Shi, Q.; Xu, J.; Wen, N. Meta-analysis of the association between serum and gingival crevicular fluid matrix metalloproteinase-9 and periodontitis. J. Am. Dent. Assoc. 2019, 150, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Andrian, E.; Grenier, D.; Rouabhia, M. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J. Dent. Res. 2006, 85, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Steinsvoll, S.; Helgeland, K.; Schenck, K. Mast cells—A role in periodontal diseases? J. Clin. Periodontol. 2004, 31, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Caraffa, A.l.; Tetè, G.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younes, A.; Emidio, P.D.; Ronconi, G. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J. Biol. Regul. Homeost. Agents 2020, 34, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Torrejon-Moya, A.; Saka-Herrán, C.; Izquierdo-Gómez, K.; Marí-Roig, A.; Estrugo-Devesa, A.; López-López, J. Oral lichen planus and Dental Implants: Protocol and Systematic Review. J. Clin. Med. 2020, 9, 4127. [Google Scholar] [CrossRef]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients: A Prospective Longitudinal Study with 1-Year Follow-Up. Clin. Implant. Dent. Relat Res. 2016, 18, 725–734. [Google Scholar] [CrossRef]
- Capparé, P.; Teté, G.; Romanos, G.E.; Nagni, M.; Sannino, G.; Gherlone, E.F. The ‘All-on-four’ protocol in HIV-positive patients: A prospective, longitudinal 7-year clinical study. Int. J. Oral Implantol. 2019, 12, 501–510. [Google Scholar]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implants 2011, 26, 866–872. [Google Scholar]
- Bruschi, G.B.; Crespi, R.; Capparè, P.; Gherlone, E. Transcrestal sinus floor elevation: A retrospective study of 46 patients up to 16 years. Clin. Implant. Dent. Relat Res. 2012, 14, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, G.B.; Crespi, R.; Capparè, P.; Bravi, F.; Bruschi, E.; Gherlone, E. Localized management of sinus floor technique for implant placement in fresh molar sockets. Clin. Implant. Dent. Relat. Res. 2013, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowińska, I.; Szyperska-Ślaska, A.; Zariczny, P.; Pasławski, R.; Kramkowski, K.; Kowalczyk, P. The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity. Materials 2021, 14, 1444. https://doi.org/10.3390/ma14061444
Rowińska I, Szyperska-Ślaska A, Zariczny P, Pasławski R, Kramkowski K, Kowalczyk P. The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity. Materials. 2021; 14(6):1444. https://doi.org/10.3390/ma14061444
Chicago/Turabian StyleRowińska, Ilona, Adrianna Szyperska-Ślaska, Piotr Zariczny, Robert Pasławski, Karol Kramkowski, and Paweł Kowalczyk. 2021. "The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity" Materials 14, no. 6: 1444. https://doi.org/10.3390/ma14061444
APA StyleRowińska, I., Szyperska-Ślaska, A., Zariczny, P., Pasławski, R., Kramkowski, K., & Kowalczyk, P. (2021). The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity. Materials, 14(6), 1444. https://doi.org/10.3390/ma14061444