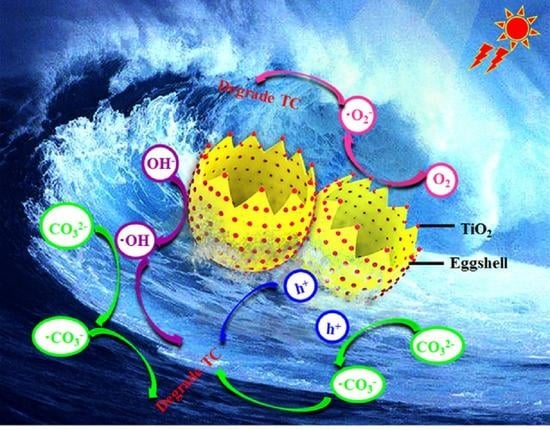
Construction of TiO2-Eggshell for Efficient Degradation of Tetracycline Hydrochloride: Sunlight Induced In-Situ Formation of Carbonate Radical
Abstract
:1. Introduction
2. Methods and Materials
2.1. Chemical and Reagents
2.2. Synthesis Process
2.3. Characterization and Analytical Methods
2.4. Photochemical Measurements
2.5. Tetracycline Hydrochloride (TC) Degradation Using Response Surface Methodology
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Wu, Y.L.; Lu, J.; Zhao, J.; Cui, J.Y.; Wu, X.L.; Yan, Y.S.; Huo, P.W. Bioinspired Synthesis of Photocatalytic Nanocomposite Membranes Based on Synergy of Au-TiO2 and Polydopamine for Degradation of Tetracycline under Visible Light. ACS Appl. Mater. Interfaces 2017, 9, 23687–23697. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, N.; Wang, P.F.; Wang, C.; Ao, Y.H. Effect of a typical antibiotic (tetracycline) on the aggregation of TiO2 nanoparticles in an aquatic environment. J. Hazard. Mater. 2018, 341, 187–197. [Google Scholar] [CrossRef]
- Liu, X.H.; Lu, S.Y.; Guo, W.; Xi, B.D.; Wang, W.L. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Le-Minh, N.; Khan, S.J.; Drewes, J.E.; Stuetz, R.M. Fate of antibiotics during municipal water recycling treatment processes. Water Res. 2010, 44, 4295–4323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Song, W.J.; Lin, H.; Wang, W.B.; Du, L.N.; Xing, W. Antibiotics and antibiotic resistance genes in global lakes: A review and meta-analysis. Environ. Int. 2018, 116, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.A.; Reddy, P.V.; Kwon, E.; Kim, K.H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef]
- Xing, Z.P.; Zhang, J.Q.; Cui, J.Y.; Yin, J.W.; Zhao, T.Y.; Kuang, J.Y.; Xiu, Z.Y.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Xiao, T.T.; Tang, Z.; Yang, Y.; Tang, L.Q.; Zhou, Y.; Zou, Z.G. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Chen, X.H.; Wu, Z.B.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus- and Sulfur-Codoped g-C3N4: Facile Preparation, Mechanism Insight, and Application as Efficient Photocatalyst for Tetracycline and Methyl Orange Degradation under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- He, X.; Fang, H.; Gosztola, D.J.; Jiang, Z.; Jena, P.; Wang, W.N. Mechanistic Insight into Photocatalytic Pathways of MIL-100(Fe)/TiO2 Composites. ACS Appl. Mater. Interfaces 2019, 11, 12516–12524. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Shi, H.M.; Ni, J.; Zheng, T.L.; Wang, X.N.; Wu, C.F.; Wang, Q.H. Remediation of wastewater contaminated by antibiotics. A review. Environ. Chem. Lett. 2019, 18, 345–360. [Google Scholar] [CrossRef]
- Isari, A.A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Rezaei Kalantary, R.; Kakavandi, B. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations: A novel hybrid system. J. Hazard. Mater. 2020, 390, 122050. [Google Scholar] [CrossRef] [PubMed]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; von Gunten, U. Photosensitizer Method to Determine Rate Constants for the Reaction of Carbonate Radical with Organic Compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. [Google Scholar] [CrossRef]
- Yadav, T.; Mungray, A.A.; Mungray, A.K. Generation of TiO2 nanoparticle-based acacia saturated eggshell bio-composite for pathogen removal. Environ. Nanotechnol. Monit. Manag. 2017, 9, 50–57. [Google Scholar]
- Liu, Y.Q.; He, X.X.; Duan, X.D.; Fu, Y.S.; Dionysiou, D.D. Photochemical degradation of oxytetracycline: Influence of pH and role of carbonate radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; Di Mento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]
- Wang, H.M.; You, C.F.; Tan, Z.C. Enhanced photocatalytic oxidation of SO2 on TiO2 surface by Na2CO3 modification. Chem. Eng. J. 2018, 350, 89–99. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.Z.; Wang, Y.T.; Jiang, Z.F.; Liu, A.N.; Pu, Y.; Xian, Q.M.; Zou, W.X.; Sun, C. Ultrafine Bi3TaO7 Nanodot-Decorated V, N Codoped TiO2 Nanoblocks for Visible-Light Photocatalytic Activity: Interfacial Effect and Mechanism Insight. ACS Appl. Mater. Interfaces 2019, 11, 13011–13021. [Google Scholar] [CrossRef]
- Wang, J.; Sun, S.J.; Pan, L.; Xu, Z.Q.; Ding, H.; Li, W. Preparation and Properties of CaCO3-Supported Nano-TiO2 Composite with Improved Photocatalytic Performance. Materials 2019, 12, 3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Q.; Qian, Q.R.; Chen, Q.; Liu, X.P.; Luo, Y.; Xue, H.; Li, Z. Significant role of carbonate radicals in tetracycline hydrochloride degradation based on solar light-driven TiO2-seashell composites: Removal and transformation pathways. Chin. J. Catal. 2020, 41, 1511–1521. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.W.; Wang, J.; Li, J.; Luan, X.; Li, Y.; Xu, R.; Wang, B. Investigation on solar photocatalytic activity of TiO2 loaded composite: TiO2/Eggshell, TiO2/Clamshell and TiO2/CaCO3. Mater. Lett. 2010, 64, 1437–144016. [Google Scholar] [CrossRef]
- Ding, Q.; Kang, Z.; Cao, L.; Lin, M.; Lin, H.; Yang, D.-P. Conversion of waste eggshell into difunctional Au/CaCO3 nanocomposite for 4-Nitrophenol electrochemical detection and catalytic reduction. Appl. Surf. Sci. 2020, 510, 145526. [Google Scholar] [CrossRef]
- Fu, X.Z.; Zeltner, W.A.; Yang, Q.; Anderson, M.A. Catalytic Hydrolysis of Dichlorodifluoromethane (CFC-12) on Sol-Gel-Derived Titania Unmodified and Modified with H2SO4. J. Catal. 1997, 168, 482–490. [Google Scholar] [CrossRef]
- Li, B.; Gan, L.; Owens, G.; Chen, Z. New nano-biomaterials for the removal of malachite green from aqueous solution via a response surface methodology. Water Res. 2018, 146, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Agarwal, S.; Asif, M.; Fakhri, A.; Sadeghi, N. Application of response surface methodology to optimize the adsorption performance of a magnetic graphene oxide nanocomposite adsorbent for removal of methadone from the environment. J. Colloid Interface Sci. 2017, 497, 193–200. [Google Scholar] [CrossRef]
- Sun, H.; He, Q.R.; She, P.; Zeng, S.; Xu, K.L.; Li, J.Y.; Liang, S.; Liu, Z.N. One-pot synthesis of Au@TiO2 yolk-shell nanoparticles with enhanced photocatalytic activity under visible light. J. Colloid Interface Sci. 2017, 505, 884–891. [Google Scholar] [CrossRef]
- Cree, D.; Rutter, A. Sustainable Bio-Inspired Limestone Eggshell Powder for Potential Industrialized Applications. ACS Sustain. Chem. Eng. 2015, 3, 941–949. [Google Scholar] [CrossRef]
- Li, Y.H.; Lv, K.L.; Ho, W.K.; Dong, F.; Wu, X.; Xia, Y. Hybridization of rutile TiO2 (rTiO2) with g-C3N4 quantum dots (CN QDs): An efficient visible-light-driven Z-scheme hybridized photocatalyst. Appl. Catal. B 2017, 202, 611–619. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Narciso-da-Rocha, C.; Polo-Lopez, M.I.; Pastrana-Martinez, L.M.; Faria, J.L.; Manaia, C.M.; Fernandez-Ibanez, P.; Nunes, O.C.; Silva, A.M.T. Solar treatment (H2O2, TiO2-P25 and GO-TiO2 photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater. Water Res. 2018, 135, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; de la Cruz, A.A.; O’Shea, K.; Falaras, P.; Dionysiou, D.D. Effects of water parameters on the degradation of microcystin-LR under visible light-activated TiO2 photocatalyst. Water Res. 2011, 45, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, X.; Nadagouda, M.N.; O’Shea, K.E.; Dionysiou, D.D. The effect of basic pH and carbonate ion on the mechanism of photocatalytic destruction of cylindrospermopsin. Water Res. 2015, 73, 353–361. [Google Scholar] [CrossRef]
- Al-Ekabi, H.; Serpone, N. Kinetics studies in heterogeneous photocatalysis. I. Photocatalytic degradation of chlorinated phenols in aerated aqueous solutions over titania supported on a glass matrix. J. Phys. Chem. 1988, 92, 5726–5731. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Ma, Y.L.; Yang, J.; Wang, L.Q.; Lv, J.M.; Ren, C.J. Aqueous tetracycline degradation by H2O2 alone: Removal and transformation pathway. Chem. Eng. J. 2017, 307, 15–23. [Google Scholar] [CrossRef]
- Yang, Y.; Zeng, Z.T.; Zhang, C.; Huang, D.L.; Zeng, G.M.; Xiao, R.; Lai, C.; Zhou, C.Y.; Guo, H.; Xue, W.J.; et al. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: Transformation pathways and mechanism insight. Chem. Eng. J. 2018, 349, 808–821. [Google Scholar] [CrossRef]
- Delépée, R.; Maume, D.; Le Bizec, B.; Pouliquen, H. Preliminary assays to elucidate the structure of oxytetracycline’s degradation products in sediments: Determination of natural tetracyclines by high-performance liquid chromatography–fast atom bombardment mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000, 748, 369–381. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.R. Preparation and characterization of nitrogen-doped TiO2/diatomite integrated photocatalytic pellet for the adsorption-degradation of tetracycline hydrochloride using visible light. Chem. Eng. J. 2016, 302, 682–696. [Google Scholar] [CrossRef]











| Factors | Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| A: Irradiation time (min) | 2 | 16 | 30 |
| B: Dosage (g∙L−1) | 0.02 | 0.1 | 0.18 |
| C: pH | 6 | 8.5 | 11 |
| D: Concentration (mg∙L−1) | 20 | 50 | 80 |
| Samples | Degradation (%) | k (min−1) | R2 |
|---|---|---|---|
| Eggshell | 63.7 | 0.0216 | 0.993 |
| TiO2 | 88.6 | 0.107 | 0.979 |
| TE | 92.0 | 0.226 | 0.999 |
| Run | A | B | C | D | TC Degradation Efficiency (%) | |
|---|---|---|---|---|---|---|
| Observed | Predicted | |||||
| 1 | 16 | 0.1 | 6 | 20 | 73.0 | 77.1 |
| 2 | 30 | 0.1 | 8.5 | 80 | 91.6 | 108.4 |
| 3 | 2 | 0.1 | 8.5 | 20 | 63.5 | 67.4 |
| 4 | 30 | 0.1 | 11 | 50 | 89.9 | 99.4 |
| 5 | 2 | 0.02 | 8.5 | 50 | 27.0 | 28.1 |
| 6 | 16 | 0.1 | 8.5 | 50 | 89.9 | 94.4 |
| 7 | 16 | 0.1 | 11 | 80 | 87.3 | 82.0 |
| 8 | 30 | 0.1 | 8.5 | 20 | 96.9 | 95.0 |
| 9 | 30 | 0.18 | 8.5 | 50 | 85.0 | 82.3 |
| 10 | 16 | 0.1 | 8.5 | 50 | 89.8 | 94.4 |
| 11 | 16 | 0.02 | 8.5 | 20 | 92.2 | 91.1 |
| 12 | 16 | 0.1 | 8.5 | 50 | 89.7 | 94.4 |
| 13 | 16 | 0.1 | 8.5 | 50 | 89.8 | 94.4 |
| 14 | 2 | 0.1 | 11 | 50 | 44.3 | 47.7 |
| 15 | 16 | 0.02 | 6 | 50 | 42.8 | 55.0 |
| 16 | 30 | 0.02 | 8.5 | 50 | 92.0 | 96.2 |
| 17 | 30 | 0.1 | 6 | 50 | 78.1 | 83.8 |
| 18 | 16 | 0.1 | 8.5 | 50 | 89.7 | 94.4 |
| 19 | 2 | 0.18 | 8.5 | 50 | 67.3 | 61.7 |
| 20 | 16 | 0.1 | 6 | 80 | 74.7 | 66.1 |
| 21 | 16 | 0.18 | 8.5 | 20 | 51.5 | 59.2 |
| 22 | 16 | 0.18 | 11 | 50 | 64.4 | 73.2 |
| 23 | 16 | 0.02 | 8.5 | 80 | 45.0 | 46.0 |
| 24 | 2 | 0.1 | 6 | 50 | 47.2 | 46.7 |
| 25 | 2 | 0.1 | 8.5 | 80 | 24.7 | 47.3 |
| 26 | 16 | 0.18 | 6 | 50 | 52.9 | 63.3 |
| 27 | 16 | 0.1 | 11 | 20 | 70.5 | 77.9 |
| 28 | 16 | 0.02 | 11 | 50 | 51.3 | 61.8 |
| 29 | 16 | 0.18 | 8.5 | 80 | 87.6 | 97.6 |
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Prob > F | ||||||
| Model | 1.17 | 14 | 0.084 | 9.7 | <0.0001 | significant |
| A | 0.56 | 1 | 0.56 | 65.02 | <0.0001 | |
| B | 0.029 | 1 | 0.029 | 3.31 | 0.0902 | |
| C | 0.013 | 1 | 0.013 | 1.47 | 0.2459 | |
| D | 0.011 | 1 | 0.011 | 1.29 | 0.2751 | |
| AB | 0.056 | 1 | 0.056 | 6.48 | 0.0233 | |
| AC | 5.32 × 10−3 | 1 | 5.32 × 10−3 | 0.62 | 0.4458 | |
| AD | 0.028 | 1 | 0.028 | 3.24 | 0.0936 | |
| BC | 2.42 × 10−4 | 1 | 2.42 × 10−4 | 0.028 | 0.8696 | |
| BD | 0.17 | 1 | 0.17 | 20.1 | 0.0005 | |
| CD | 5.70 × 10−3 | 1 | 5.70 × 10−3 | 0.66 | 0.4303 | |
| A2 | 0.073 | 1 | 0.073 | 8.47 | 0.0114 | |
| B2 | 0.18 | 1 | 0.18 | 20.88 | 0.0004 | |
| C2 | 0.14 | 1 | 0.14 | 15.78 | 0.0014 | |
| D2 | 0.012 | 1 | 0.012 | 1.36 | 0.2635 | |
| Residual | 0.12 | 14 | 8.64 × 10−3 | |||
| Lack of Fit | 0.12 | 10 | 0.012 | |||
| Pure Error | 0 | 4 | 0 | |||
| Cor Total | 1.29 | 28 | ||||
| R2 | 0.906 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Wang, J.; Yang, M.-Q.; Qian, Q.; Liu, X.-P.; Xiao, L.; Xue, H. Construction of TiO2-Eggshell for Efficient Degradation of Tetracycline Hydrochloride: Sunlight Induced In-Situ Formation of Carbonate Radical. Materials 2021, 14, 1598. https://doi.org/10.3390/ma14071598
Huang Z, Wang J, Yang M-Q, Qian Q, Liu X-P, Xiao L, Xue H. Construction of TiO2-Eggshell for Efficient Degradation of Tetracycline Hydrochloride: Sunlight Induced In-Situ Formation of Carbonate Radical. Materials. 2021; 14(7):1598. https://doi.org/10.3390/ma14071598
Chicago/Turabian StyleHuang, Zhuquan, Jiaqi Wang, Min-Quan Yang, Qingrong Qian, Xin-Ping Liu, Liren Xiao, and Hun Xue. 2021. "Construction of TiO2-Eggshell for Efficient Degradation of Tetracycline Hydrochloride: Sunlight Induced In-Situ Formation of Carbonate Radical" Materials 14, no. 7: 1598. https://doi.org/10.3390/ma14071598
APA StyleHuang, Z., Wang, J., Yang, M. -Q., Qian, Q., Liu, X. -P., Xiao, L., & Xue, H. (2021). Construction of TiO2-Eggshell for Efficient Degradation of Tetracycline Hydrochloride: Sunlight Induced In-Situ Formation of Carbonate Radical. Materials, 14(7), 1598. https://doi.org/10.3390/ma14071598