Influence of Cold Deformation on Carbide Precipitation Kinetics in a Fe-22Mn-0.45C TWIP Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results
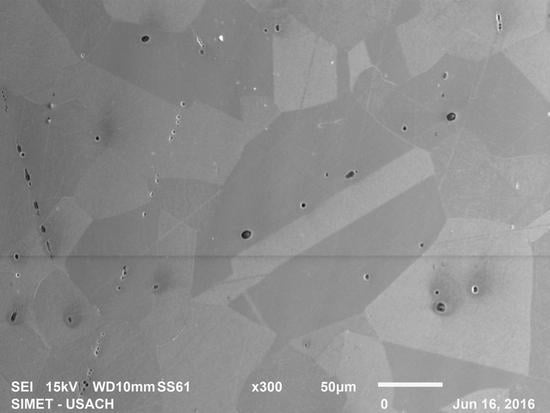
3.1. Metallographic Analysis
3.2. Identification of Fe-Mn-Carbides
3.3. Measurement of the Volumetric Fraction of Fe-Mn-Carbides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Cooman, B.C.; Estrin, Y.; Kim, S.K. Twinning-induced plasticity (TWIP) steels. Acta Mater. 2018, 142, 283–362. [Google Scholar] [CrossRef]
- Kestenbach, H.J.; Rodrigues, J.A.; Dermonde, J.R. Niobium carbonitride precipitation in low carbon high manganese steel after hot rolling. Mater. Sci. Technol. 1989, 5, 29–35. [Google Scholar] [CrossRef]
- Lis, J.; Lis, A.; Kolan, C. Manganese partitioning in low carbon manganese steel during annealing. Mater. Charact. 2008, 59, 1021–1028. [Google Scholar] [CrossRef]
- Di Martino, S.F.; Thewlis, G. Transformation characteristics of ferrite/carbide aggregate in continuously cooled, low carbon-manganese steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 579–594. [Google Scholar] [CrossRef]
- Frommeyer, G.; Brüx, U.; Neumann, P. Supra-Ductile and High-Strength Manganese-TRIP/TWIP Steels for High Energy Absorption Purposes. ISIJ Int. 2003, 43, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Bouaziz, O.; Guelton, N. Modelling of TWIP effect on work-hardening. Mater. Sci. Eng. A 2001, 319–321, 246–249. [Google Scholar] [CrossRef]
- Prakash, A.; Hochrainer, T.; Reisacher, E.; Riedel, H. Twinning Models in Self-Consistent Texture Simulations of TWIP Steels. Steel Res. Int. 2008, 79, 645–652. [Google Scholar] [CrossRef]
- Allain, S.; Chateau, J.-P.; Dahmoun, D.; Bouaziz, O. Modeling of mechanical twinning in a high manganese content austenitic steel. Mater. Sci. Eng. A 2004, 387–389, 272–276. [Google Scholar] [CrossRef]
- Ding, H.; Tang, Z.-Y.Y.; Li, W.; Wang, M.; Somg, D. Microstructures and Mechanical Properties of Fe-Mn-(Al, Si) TRIP/TWIP Steels. J. Iron Steel Res. Int. 2006, 13, 66–70. [Google Scholar] [CrossRef]
- Bouaziz, O.; Allain, S.; Scott, C. Effect of grain and twin boundaries on the hardening mechanisms of twinning-induced plasticity steels. Scr. Mater. 2008, 58, 484–487. [Google Scholar] [CrossRef]
- Wittig, J.E.; Pozuelo, M.; Jiménez, J.A.; Frommeyer, G. Temperature dependent deformation mechanisms of a high nitrogen-manganese austenitic stainless steel. Steel Res. Int. 2009, 80, 66–70. [Google Scholar]
- Phiu-on, K.; Bleck, W.; Schwedt, A.; Mayer, J. Effects of Solution Treatment and Test Temperature on Tensile Properties of High Mn Austenitic Steels. Steel Res. Int. 2009, 80, 29–38. [Google Scholar]
- Bracke, L.; Kestens, L.; Penning, J. Transformation mechanism of α′-martensite in an austenitic Fe-Mn-C-N alloy. Scr. Mater. 2007, 57, 385–388. [Google Scholar] [CrossRef]
- Galán, J.; Samek, L.; Verleysen, P.; Verbeken, K.; Houbaert, Y.; Houbaert, Y. Advanced high strength steels for automotive industry. Rev. Metal. 2012, 48, 118–131. [Google Scholar] [CrossRef]
- Hamada, A.S.S.; Karjalainen, L.P.P.; Somani, M.C.C. The influence of aluminum on hot deformation behavior and tensile properties of high-Mn TWIP steels. Mater. Sci. Eng. A 2007, 467, 114–124. [Google Scholar] [CrossRef]
- Ha, Y.; Kim, H.; Kwon, K.H.; Lee, S.-G.; Lee, S.; Kim, N.J. Microstructural Evolution in Fe-22Mn-0.4C Twinning-Induced Plasticity Steel During High Strain Rate Deformation. Metall. Mater. Trans. A 2015, 46, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Hamada, A.S.; Karjalainen, L.P.; Puustinen, J. Fatigue behavior of high-Mn TWIP steels. Mater. Sci. Eng. A 2009, 517, 68–77. [Google Scholar] [CrossRef]
- Mendes Rodrigues, M.; Gouveia, H.; Smith, A.; Pratolongo, G.; Karjalainen, P.; Sevillano, J.G.; De las Cuevas, F.; Ferraiuolo, A. Metallurgical Design of High-Strength Austenitic Fe-C-Mn Steels with Excellent Formability (Metaldesign); European Union: Luxembourg, 2012. [Google Scholar]
- Bouaziz, O.; Zurob, H.; Chehab, B.; Embury, J.D.; Allain, S.; Huang, M. Effect of chemical composition on work hardening of Fe-Mn-C TWIP steels. Mater. Sci. Technol. 2011, 27, 707–709. [Google Scholar] [CrossRef]
- Scott, C.; Allain, S.; Faral, M.; Guelton, N.; Allain, S.; Faral, M. The development of a new Fe-Mn-C austenitic steel for automotive applications. Rev. Métallurgie 2006, 103, 293–302. [Google Scholar] [CrossRef]
- Kang, S.; Jung, Y.S.; Jun, J.H.; Lee, Y.K. Effects of recrystallization annealing temperature on carbide precipitation, microstructure, and mechanical properties in Fe-18Mn-0.6C-1.5Al TWIP steel. Mater. Sci. Eng. A 2010, 527, 745–751. [Google Scholar] [CrossRef]
- Scott, C.; Remy, B.; Collet, J.-L.; Cael, A.; Bao, C.; Danoix, F.; Malard, B.; Curfs, C. Precipitation strengthening in high manganese austenitic TWIP steels. Int. J. Mater. Res. 2011, 102, 538–549. [Google Scholar] [CrossRef]
- Cancino Serrano, M.J. Estudio de Soldabilidad de Aceros TWIP 0.3%C, 22%Mn, 0.2%Si Mediante Sistema TIG; Universidad de Santiago de Chile: Santiago, Chile, 2016. [Google Scholar]
- ASTM E415-21; Standard Test Method for Analysis of Carbon and Low-Alloy Steel by Spark Atomic. American Society for Testing and Materials: West Conshohocken, PA, USA, 2015; Volume 1999, pp. 1–9.
- Available online: http://www.mediacy.com/imageproplus (accessed on 18 April 2017).
- ASTM E384-17; Standard Test Method for Microindentation Hardness of Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016; pp. 1–42.
- Kohout, J. An alternative to the JMAK equation for a better description of phase transformation kinetics. J. Mater. Sci. 2008, 43, 1334–1339. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Klinkenberg, C.; Hulka, K.; Bleck, W. Niobium carbide precipitation in microalloyed steel. Steel Res. Int. 2004, 75, 744–752. [Google Scholar] [CrossRef]
- De Las Cuevas, F.; Aguilar, C.; Sevillano, J.G. A further study of the kinetics of recrystallization and grain growth of cold rolled TWIP steel. Rev. Metal. 2018, 54, 4. [Google Scholar]
- Lothongkum, G.; Ratanamahasakul, S.; Wangyao, P. Effect of heat treatment conditions on dynamic parameters of secondary carbide precipitation in centrifugally cast iron-base superalloy. Acta Metall. Slovaca 2005, 11, 54–61. [Google Scholar]
- Leslie, W.C. Physical Metallurgy of Steels, 1st ed.; McGraw-Hill College: New York, NY, USA, 1981. [Google Scholar]












| %C | %Mn | %Si | %Cr | %P | %S | %Fe |
|---|---|---|---|---|---|---|
| 0.45 | 21.94 | 0.21 | 0.16 | 0.008 | 0.002 | balance |
| (a) Stage 1 | |||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 525 | 550 | 575 | 600 | 625 | 650 | |
| 40% cold-deformation | k | 1.31 | 1.15 | 1.01 | - | - | - |
| Q | 408.93 kJ/mol | ||||||
| B0 | 4.77 × 1017 | ||||||
| 60% cold-deformation | k | 1.46 | 1.34 | 1.25 | - | - | - |
| Q | 326.38 kJ/mol | ||||||
| B0 | 1.48 × 1012 | ||||||
| 80% cold-deformation | k | 1.68 | 1.56 | 1.44 | 1.30 | 1.12 | 0.82 |
| Q | 254.43 kJ/mol | ||||||
| B0 | 1.62 × 107 | ||||||
| (b) Stage 2 | |||||||
| Temperature (°C) | 525 | 550 | 575 | 600 | 625 | 650 | |
| 40% cold-deformation | k | - | - | - | 0.82 | 0.60 | 0.54 |
| Q | 154.80 kJ/mol | ||||||
| B0 | 5.46 × 108 | ||||||
| 60% cold-deformation | k | - | 0.84 | 0.83 | 0.73 | 0.67 | 0.47 |
| Q | 104.14 kJ/mol | ||||||
| B0 | 2.12 × 1000 kJ/mol | ||||||
| 80% cold-deformation | k | 0.76 | 0.75 | 0.63 | 0.56 | 0.45 | 0.22 |
| Q | 78.85 kJ/mol | ||||||
| B0 | 6.73 × 10−1 | ||||||
| Annealing Temperature (°C) | Austenite Grain Size (μm) | ||
|---|---|---|---|
| 40% Deformation | 60% Deformation | 80% Deformation | |
| 525 | 1.76 | 0.87 | |
| 550 | 1.98 | 1.30 | |
| 575 | 3.68 | 2.65 | 2.48 |
| 600 | 4.00 | 3.78 | 3.52 |
| 625 | 7.52 | 6.49 | 5.27 |
| 650 | 11.51 | 9.24 | 7.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobar, J.; Jiménez, J.L.; Artigas, A.; Perez-Ipiña, J.; Monsalve, A. Influence of Cold Deformation on Carbide Precipitation Kinetics in a Fe-22Mn-0.45C TWIP Steel. Materials 2022, 15, 3748. https://doi.org/10.3390/ma15113748
Escobar J, Jiménez JL, Artigas A, Perez-Ipiña J, Monsalve A. Influence of Cold Deformation on Carbide Precipitation Kinetics in a Fe-22Mn-0.45C TWIP Steel. Materials. 2022; 15(11):3748. https://doi.org/10.3390/ma15113748
Chicago/Turabian StyleEscobar, Javier, José Luis Jiménez, Alfredo Artigas, Juan Perez-Ipiña, and Alberto Monsalve. 2022. "Influence of Cold Deformation on Carbide Precipitation Kinetics in a Fe-22Mn-0.45C TWIP Steel" Materials 15, no. 11: 3748. https://doi.org/10.3390/ma15113748
APA StyleEscobar, J., Jiménez, J. L., Artigas, A., Perez-Ipiña, J., & Monsalve, A. (2022). Influence of Cold Deformation on Carbide Precipitation Kinetics in a Fe-22Mn-0.45C TWIP Steel. Materials, 15(11), 3748. https://doi.org/10.3390/ma15113748